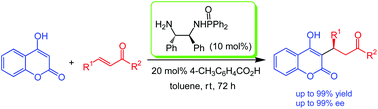
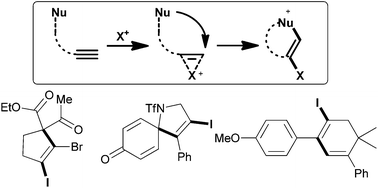
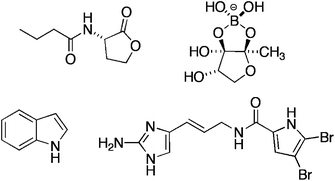
 Beers, wines and cheeses are enjoyed around the world today and have been for millennia. In fact the practices of brewing and cheese-making pre-date recorded history so it is difficult to accurately determine when we first started using naturally occurring enzymes and microorganisms to create valuable (and in this case, tastier!) products.
Beers, wines and cheeses are enjoyed around the world today and have been for millennia. In fact the practices of brewing and cheese-making pre-date recorded history so it is difficult to accurately determine when we first started using naturally occurring enzymes and microorganisms to create valuable (and in this case, tastier!) products.
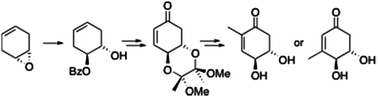
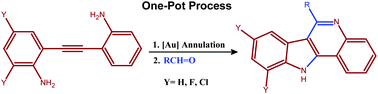
Biocatalysts are of course used in far more diverse applications than the creation of food-stuffs, including in many organic syntheses and in the generation of fine chemicals. Due to their natural design, they can offer superior selectivity for particular products and have a far lower environmental impact than many traditional catalysts. Our knowledge and understanding of biocatalysts has increased dramatically in the last few decades, which has allowed us to develop biologically modified and biomimetic catalysts for a range of applications.
To keep you up to date with the latest advances in this rapidly expanding field we have collected together these high impact articles and made them free to access until the 31st October!