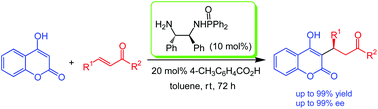
The article features a new kind of bifunctional primary amine–phophinamide catalyst, which when applied to catalyze the Michael reaction of 4-hydroxycoumarin to α,β-unsaturated ketones lead to the synthesis of the important pharmaceutical product Warfarin.
Highly enantioselective synthesis of Warfarin and its analogs catalysed by primary amine–phosphinamide bifunctional catalysts
Juan Dong and Da-Ming Du
DOI: 10.1039/C2OB26334C
| This article features as part of OBC‘s organocatalysis collection. If you liked this article why not have a look at the collection for other articles on organocatalysis…. |










