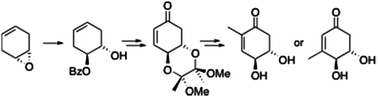
In this Communication Peter O’Brien and colleagues from the University of York describe a short and efficient asymmetric synthesis of a chiral cyclohexenone derivative ((4S,5S)-dihydroxycyclohexen-1-one ). This derivative is a very useful synthon for a variety of natural products, as shown by O’Brien et al., who prepare 2 cyclohexenone natural products isolated from the mycelia of Lasiodiplodia theobromae.
The approach presented has been described as being ‘elegant chemistry which uses symmetry breaking reactions to quickly reach a synthetic goal.’
Find out more by downloading the Communication now….
Catalytic asymmetric synthesis of butane diacetal-protected (4S,5S)-dihydroxycyclohexen-1-one and use in natural product synthesis
David J. Burns, Shuji Hachisu, Peter O’Brien and Richard J. K. Taylor
DOI: 10.1039/C2OB26406D