“It’s cold and white everywhere. What else can you expect on early January’s very snowy evening!” I mumbled to myself and was heading towards home exhausted when I witnessed the almost ungovernable sliding inevitable collision of two nice looking vehicles with people on driving seats trying hard to salvage the situation. It was not a gratifying view for the spectators let alone for the vehicle owners and insurance companies (of course). Knowing that not much could be done from my side, I resumed my meticulous “frictionless” walk but this time pondering over the collisions.
In general as the scale (size) of the colliding bodies increases, the collision turns out to be more and more disastrous, collision of the bodies the scale of aircraft is unimaginably catastrophic with the scale of the damage shrinking with the scale of the colliding bodies. The thought which struck was, is there any collision which can be useful rather than damaging? Without wasting a second, I questioned myself with conquering smirk, how about collisions of molecular levels?! “They are awesome and intriguing!” was my first reaction. Yes, molecules of angstrom meter (10000 times smaller that human hair diameter) size collide with each other in solution. Not only do they collide with each other, they construct a multitude of stunning phenomena.
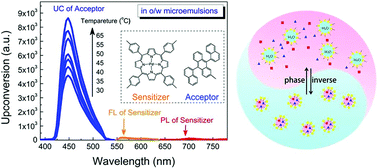
With this same collision, some class of substances give rise to the phenomenon of light upconversion (converting light from low energy to high energy). Typical upconversion systems consists of 2 dyes (highly light responsive substances). When light of particular energy (for example, green light) is irradiated on the system, one of the dyes (donor) is excited by the absorption of the light. 2 molecules of the donor dye collide and transfer their energy to 2 molecules of the other dye (acceptor) present in the system. A further 2 molecules of the acceptor dye collide with each other and emit colorful light (fluorescence) of a different colour (for example: blue, a higher energy). As light emitted from system is of a higher energy than the light provided to system, its called an upconversion system. Collision on a molecular level can do very fun things.
These kind of upconversion systems can have applications in solar cells and for that, a brief and detailed study of these kind of systems is necessary. Generally it is difficult to study these systems in the water phase because the dyes involved hate water. Chenquing etal successfully stabilized these systems in nanopockets of Tween 20 (surfactant) micelles in water. They also studied upconversion systems based on different kinds of acceptors and donors dyes. This study, which is cited at the end, not only correlates the structure of the dyes to the upconversion efficiency but also demonstrates effect of temperature on the amount of light drawn out of the system. Excellent efforts for exploiting this intriguing phenomenon in photochemistry (producing electricity using light emitted) are also illustrated. This study also claims to be the first study to provide clean upconversion without degassing.
Oil-in-water microemulsion: an effective mediumfor triplet–triplet annihilated upconversion withefficient triplet acceptors.
Changqing Ye, Bao Wang, Roukang Hao, Xiaomei Wang, Ping Ding, Xutang Tao, Zhigang Chen, Zuoqin Lianga and Yuyang Zhoua
J. Mater. Chem. C, 2014, Advance Article
DOI: 10.1039/C4TC00791C
Padmanabh Joshi is a guest web writer for the Journal of Materials Chemistry blog. He currently works at the Department of Chemistry, University of Cincinnati.
To keep up-to-date with all the latest research, sign-up to our RSS feed or Table of contents alert.