The 3rd Asia-Oceania Conference on Green and Sustainable Chemistry (AOC-3) will be held in Melbourne, Australia on December 4 – 7, 2011. The aim of the conference is to provide a platform for interaction and exchange of ideas between practitioners in Green Chemistry, and to promote Green Chemistry in the Asia-Oceania region. Program highlights will include presentations by the ‘father of green chemistry’, Dr Paul Anastas and 2010 Nobel Prize Winner, Dr Akira Suzuki. Abstract submission is still open for oral consideration and earlybird rates are available. Please visit http://www.greenoz2011.org/ to register and lodge your abstract!
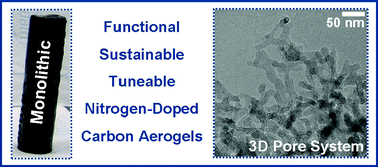
Sustainable synthesis of nitrogen-doped carbon aerogels
Scientists from Germany and Japan have developed a method to synthesize nitrogen-doped carbon aerogels (CA) from sustainable starting materials.
Aerogels are coherent, highly porous solid materials with attractive physical properties such as low density, excellent mass-transfer properties, low thermal conductivity, and low dielectric permittivity. Previous methods to synthesize nitrogen-doped CA have several disadvantages, as the materials are phenol-based with low conductivity and relatively inert, making post-chemical modification challenging.
To read the full article, please click on the link below! This article is free to access until September 6th.
A sustainable synthesis of nitrogen-doped carbon aerogels, Robin J. White, Noriko Yoshizawa, Markus Antonietti and Maria-Magdalena Titirici, Green Chem., 2011, DOI: 10.1039/C1GC15349H
Green Chemistry Volume 13 Issue 8 online now!
Green Chemistry issue 8 features an artist’s impression* of the problematic situation of Fischer-Tropsch bimetallic catalysis, it highlights a critical review from Advisory Board member Gadi Rothenberg and co-workers from The Netherlands and France. Their short critical review summarises and analyses the developments in Fischer–Tropsch catalysis using bimetallic alloys, read the full article online here.
 The inside front cover highlights the article ‘Controlled polymerisation of lactide using an organo-catalyst in supercritical carbon dioxide’ by Idriss Blakey and co-workers from the University of Queensland, Australia and the University of Nottingham, UK. Their article, previously highlighted on this blog, reports the ‘green’ synthesis of well defined polylactic acid (PLA) via organo-catalysis, without using any organic solvents. Read the full article online here.
The inside front cover highlights the article ‘Controlled polymerisation of lactide using an organo-catalyst in supercritical carbon dioxide’ by Idriss Blakey and co-workers from the University of Queensland, Australia and the University of Nottingham, UK. Their article, previously highlighted on this blog, reports the ‘green’ synthesis of well defined polylactic acid (PLA) via organo-catalysis, without using any organic solvents. Read the full article online here.
*The copyright owner, Prof. Dr. Gadi Rothenberg, hereby gives all persons permission to use this cover image for any purpose, provided that the artist’s name and website is correctly cited. Drawn by Itamar Daube; http://www.itamardaube.com
How to design a safer chemical
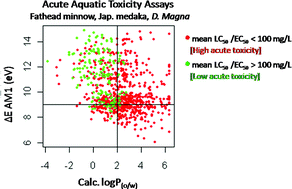 A recent Green Chemistry article from Julie Zimmerman, Paul Anastas and co-workers from Yale University and Baylor University describes guidelines which should be followed in order to design chemicals with reduced aquatic toxicity. Their article has been highlighted in Nature News.
A recent Green Chemistry article from Julie Zimmerman, Paul Anastas and co-workers from Yale University and Baylor University describes guidelines which should be followed in order to design chemicals with reduced aquatic toxicity. Their article has been highlighted in Nature News.
The team highlight that there is a need for synthetic chemists to focus on design of safer chemicals rather than testing for toxicity after production. The team explored mechanistically-driven qualitative and quantitative analyses between the in-silico predicted molecular properties and in vivo toxicity data to propose property limits associated with higher probabilities of safe chemicals. They propose design guidelines that can be used to significantly increase the probability that a chemical will have low toxicity to the aquatic species studied.
Interested in knowing more? Read the full article for free until September 1st!
Towards rational molecular design: derivation of property guidelines for reduced acute aquatic toxicity
Adelina M. Voutchkova, Jakub Kostal, Justin B. Steinfeld, John W. Emerson, Bryan W. Brooks, Paul Anastas and Julie B. Zimmerman
Green Chem., 2011, DOI: 10.1039/C1GC15651A
Sustainable recovery of pure natural vanillin from fermentation media in one step
Pure vanillin can be recovered from fermentation media in a single, sustainable, solvent-free pervapouration step.
Vanillin is one of the world’s most important aroma compounds in the profitable market of flavours and fragrances. The majority of vanillin is produced chemically, with only a very small proportion extracted from beans. Vanillin can 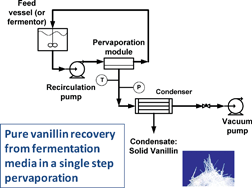
In this work Crespo and co-workers have used an organophilic pervaporation method to recover vanillin from fermentation media. This process involves a hydrophobic non-porous membrane in which hydrophobic solutes sorb very favourably to it, diffuse across the membrane and desorb under stimulus (e.g. changes in pressure). This method avoids the use of organic solvents and contamination from adsorbents, and provided quantitative recovery of vanillin.
To find our more, just click the link below! This article is free to access until 29th August!
Sustainable recovery of pure natural vanillin from fermentation media in a single pervaporation step, Carla Brazinha, Dalje S. Barbosa and João G. Crespo, Green Chem., 2011, DOI: 10.1039/C1GC15308K
Granting wishes for researchers
Rafael Luque discusses funding for early stage researchers and the importance of green chemistry with Anna Simpson

Rafael Luque is the Ramon y Cajal fellow at the University of Cordoba in Spain. His interests range from materials science, nanotechnology and heterogeneous catalysis to biomass valorisation and biofuels.
How did you get to where you are today?
I was always fascinated by chemistry because it’s in everything. This table that we are sitting at is chemistry!
I did my undergraduate degree at the University of Cordoba in Spain and I was delighted to receive a grant to do my PhD in the organic chemistry department there. I started to have my own ideas and in pursuing them, my supervisors were always happy. It all worked out really well in the end!
During my PhD, I spent six months at the green chemistry centre of excellence at the University of York in the UK. I worked with Duncan Macquarrie who had a big impact on my career. I am very grateful to him and to James Clark for this. After my PhD, I returned to York as a green chemistry research associate and spent some of the most wonderful years of my career to date there. Everything went very well in York; however, after three and a half years as a postdoc, I needed to move on with my career and my life. So, due to personal and family pressures, I decided to move back to Cordoba. In the beginning, this was difficult but I managed to get a nice fellowship and now have a small group of four PhD students and a postdoc starting soon.
What are the biggest challenges facing young researchers and what’s your advice for someone about to embark on the next step after a PhD or postdoc?
Innovation is the key to success. You can’t get grant money for doing the same thing that we have been doing for over 20 years, it must be new work.
Getting funding and grants is challenging, but even with a very small group, such as one masters or PhD student, you can start to do more work. I have met lots of innovative and creative people in Europe and Spain. They have lots of interesting ideas and promising research but the problem is they need basic funding to progress. My advice is to keep trying and not lose courage as generally, if you are unsuccessful, you have to carry on and fight for what you want to do.
You have quite wide ranging research interests but the theme that underpins it all is green and sustainable chemistry. Why is green chemistry important?
Green chemistry is going to be everything in the future. We will be using green products made using technology with low environmental impact and utilising locally sourced materials and even waste. The perception of waste as a resource rather than a problem is something we have to change in people’s minds. We still have some work to do there.
After working in both the UK and Spain, what differences did you experience between the chemical research communities in these countries?
There are lots of differences. In the UK, there are big funding agencies to support researchers. This is something we lack in Spain, but I must stress that in Spain, I have found that we are good and very competitive in terms of publications and research. We are innovative and creative and I was very pleased to see, when I got back, that there were so many people doing such a great job – it is a big inspiration for me. It is especially apparent with young researchers. They lack resources but are still self-motivated to pursue their dreams, which is very encouraging. I take care of students and encourage them to carry on because I can see that this degree of motivation is getting them everywhere. I love teaching – there is always a way to engage the students to learn about chemistry. Being a young academic often helps because students feel closer to you as there is not an age barrier. The language and the way you communicate with the students are not so different.
Where does your funding come from?
At the moment it’s mostly national and regional funding. This wasn’t particularly easy to get, but the government supports young researchers with groundbreaking ideas. The funding I received is aimed at early career researchers and for me, after proposing one of these groundbreaking concepts, it was helpful that I could get some funding – it allowed me to go back to Cordoba.
Hopefully, at the end of October, I will try my best to be successful with a European bid, which could potentially put me in a more stable situation and allow me to grow a bigger group. This funding, called a ‘Starting Research Grant’ is indeed a lot of money – 1.5 million Euros – so it could have a big impact on my career. Its purpose is to allow someone to develop ideas and start an independent research group that could lead to important developments for Europe. We plan to work with lignocellulosics and lignin, one of our main targets.
What do you like to do when you are not doing chemistry?
I love travelling – it’s one of my favourite things, so I have been all over the world except for Australia and New Zealand. When I’m at home, I’m addicted to video games; I must admit that I am a big fan. Other than that, I love music, so if I wasn’t a chemist I would probably be a DJ.
Read some of Rafael Luque’s latest work in Green Chemistry by following the links below:
Catalytically active self-assembled silica-based nanostructures containing supported nanoparticles
Camino Gonzalez-Arellano, Alina Mariana Balu, Rafael Luque and Duncan J. Macquarrie
Green Chem., 2010, 12, 1995-2002
Magnetically separable nanoferrite-anchored glutathione: aqueous homocoupling of arylboronic acids under microwave irradiation
Rafael Luque, Babita Baruwati and Rajender S. Varma
Green Chem., 2010, 12, 1540-1543
Highly active and selective supported iron oxide nanoparticles in microwave-assisted N-alkylations of amines with alcohols
Camino Gonzalez-Arellano, Kenta Yoshida, Rafael Luque and Pratibha L. Gai
Green Chem., 2010, 12, 1281-1287
Efficient and recyclable catalysts for reactions with biomass-derived products
Hydrolytic hydrogenation of cellulose with hydrotreated caesium salts of heteropoly acids and Ru/C. 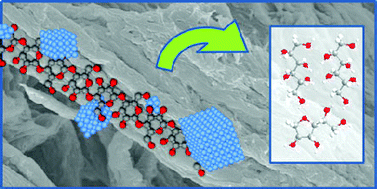
However, in this work Sels and co-workers have found that the caesium salts of HPAs are not only highly selective (giving up to 90% yields of hexitols) and can be performed under mild reaction conditions, the Cs HPA salts could be recovered by simple recrystallisation at room temperature without using organic solvents. (Green Chem., 2011, DOI: 10.1039/c1gc15350a)
Selective oxidation of 5-hydroxymethyl-2-furfural using supported gold-copper nanoparticles. 5-Hydroxymethyl-2-furfural (HMF), formed from the dehydration of sugars, can be oxidised to 2,5-furandicarboxylic acid (FDCA), which recently has been suggested as a substitute for terephthalate acid – the monomer for the 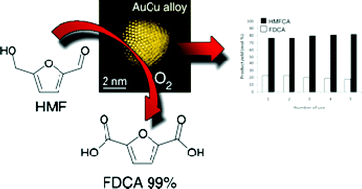
In this work Hutchings and colleagues report the use of gold-copper supported nanoparticles as an effective catalyst for the oxidation of HMF to FDCA. Although supported gold nanoparticles have been applied to this reaction previously, catalyst stability has remained very low. However, the bimetallic nanoparticles reported here, supported on titania, exhibit a remarkable degree of stability, even in the presence of base. The catalyst could be recovered by filtration and reused several times without significant loss of activity. (Green Chem., 2011, DOI: c1gc15355b)
Enzymatic reductions for chemists
Reduction reactions with enzymes has developed rapidly in the past few years. Previously, biocatalytic reductions have been challenging due to the dependency of the enzyme on a co-factor, narrow substrate range and restrictions to reactions in aqueous media. However, the majority of these challenges have now been, or are about to be, solved.
In this review article, Hollmann and co-workers give an overview of the recent developments in biocatalytic reduction, with a critical view on the green aspects. To read more, please read the full article, which is free until 12 August, by clicking the link below.
Enzymatic reductions for the chemist, Frank Hollmann, Isabel W. C. E. Arends and Dirk Holtmann, Green Chem., 2011, DOI: 10.1039/C1GC15424A
You may also be interested in the following review, free until 12 August:
Enzyme-mediated oxidations for the chemist, Frank Hollmann, Isabel W. C. E. Arends, Katja Buehler, Anett Schallmey and Bruno Bühler, Green Chem., 2011, 13, 226-265
Utilizing natural resources – ethenolysis of natural rubber and pyrolysis of macro-algae
| On the ethenolysis of natural rubber and squalene. Plenio and Wolf report the utilisation of natural rubber and squalene to obtain a set of terminal olefins. The main focus for producing olefins from renewable resources has been on plant oils. Despite the fact that the annual production (2007) of natural rubber was 9.7 × 106 tons, this polyolefin has not been widely used. However, due to the high degree of stereoregularity (in terms of double bond geometry and orientation) of natural rubber and squalene, Plenio was able to obtain controlled polymer degradation which resulted in the synthesis of sveral small oligoisoprenes. Future work is going to be directed scaling up this reaction and ultizing the products for the synthesis of flavours, odorants, etc. (Green Chem., 2011, DOI: 10.1039/c1gc15265c) | 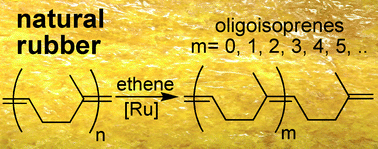 |
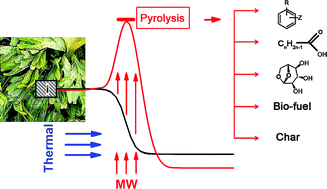 |
Microwave-mediated pyrolysis of macro-algae. Macro-algae is an abundant but generally underutilised resource – currently less that 1% is used. Its integration into a biofrinery is challenging as it contains increased levels of halogenated compounds, alkali earth and heavy metals which restrict its use in direct combustion. In this work, Clark and co-workers report the effective pyrolysis of macro-algae using a microwave. Pyrolysis is an established technique for deconstructing biomass using heat under an inert atmosphere to obtain high-value chemicals and platform molecules. In this study chemical rich bio-oils were obtained from macro-algae at temperatures lower than those used for conventional biomass and by using microwave irradiation they obtained a higher yield (21%) than that obtained with conventional heating (14%). (Green Chem., 2011, DOI: 10.1039/c1gc15560a) |
Critical reviews on selective oxidation of glycerol and recycling homogeneous catalysts using mebrane separation.
| Selective catalytic oxidation of glycerol – perspectives for high value chemicals. The increasing worldwide production of biodiesel has led to an excess supply of crude glycerol, prompting researchers to investigate its valorisation. Thanks to its three hydroxyl groups, glycerol is a potential starting material for several high-value fine chemicals. Various metals can catalyse glycerol oxidation. However, the selectively of these reactions is dependent on the active phase, metal particle size, pore size of the support and pH of the reaction medium. In this review, Dumeignil and co-workers look at the recent developments in new catalysts and spotlight the role of reaction conditions. (Green Chem., 2011, DOI: 10.1039/c1gc15320j) | 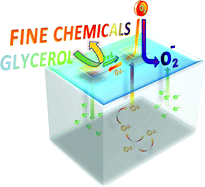 |
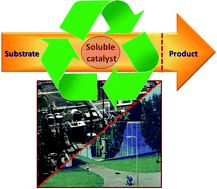 |
Recent advances in recycling of homogeneous catalysts using membrane separation. Membrane filtration is now an attractive approach for the recycling of soluble catalysts. Nanofiltration has shown great potential as a method for process intensification in organo-, anzyme, and homogeneous catalysis. Vogt and co-workers discuss selected, recent advances in catalyst recovery by membrane filtration in this review and look at implications for future development. (Green Chem., 2011, DOI: 10.1039/c1gc15264e) |











