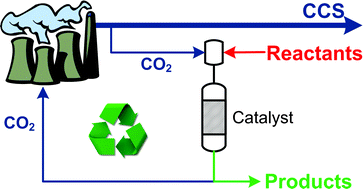
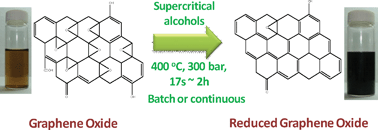
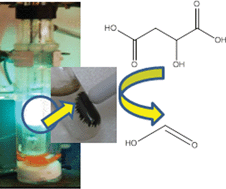
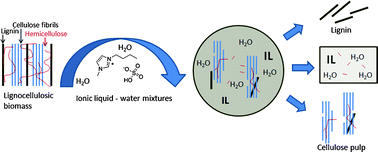
8th GC&C Symposium on Food Waste Utilisation (18th October, 10:00—17.30, The King’s Manor, York)
The Green Chemistry and the Consumer annual symposia bring together a diverse audience of representatives from consumer product supply chains, academics, NGOs, government, trade associations, media and other stakeholders to learn about green chemistry solutions for sustainable product supply chains. The 8th symposium, ‘Waste to Wealth: Food Waste Utilisation’, will explore recent advances in the field, including new technologies for food waste valorisation. The programme for the day will consist of a blend of both presentations and breakout sessions. The symposium will conclude with a wine reception providing a further networking opportunity.
Speakers include:
- Malcolm Bailey, Regional Director of NISP
- Prof. Robert Edwards, Chief Scientist, FERA
- Dr. John Williams, Head of Materials for Energy and Industry, NNFCC
Who should attend?
These events are open to all and are aimed at organisations and individuals with an interest in green chemistry and sustainable chemical products. The event will provide an invaluable opportunity for mutual learning and technology transfer.
This event will be of interest to:
- Food producers
- Food retailers
- Energy providers
- Waste managers and regulators
- Bio-based manufacturers
For more details about the conference including registration, please contact Heather Hamilton (Green Chemistry Network Manager) at heather.hamilton@greenchemistrynetwork.org.