Scientists have developed a new strategy for grinding wood chips which leads to significant energy savings.
Wood is a source of biomass that can be converted into renewable fuels and chemicals. However, it has been noticed that in pretreatment processes, the size of the wood chips or particles used affects their rates of dissolution. Therefore most studies to-date have used pre-ground biomass, but the energy consumption for grinding untreated wood to powder is high and could be a process-limiting cost for the production of renewable chemicals and fuels.
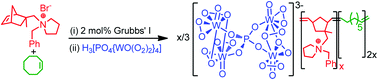
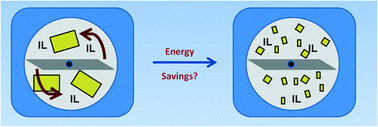 Tom Welton and colleagues from Imperial College London, UK, and the University of Natural Resources and Life Sciences, Vienna, Austria, have developed a new process where an ionic liquid is used to soak the wood chips in prior to grinding. This step gave significant energy savings which was shown to be due to the lubricating properties of the ionic liquid rather than any physico-chemical modifications of the biomass.
Tom Welton and colleagues from Imperial College London, UK, and the University of Natural Resources and Life Sciences, Vienna, Austria, have developed a new process where an ionic liquid is used to soak the wood chips in prior to grinding. This step gave significant energy savings which was shown to be due to the lubricating properties of the ionic liquid rather than any physico-chemical modifications of the biomass.
This article is free to access until the 10th April 2012! Click on the link below to find out more…
Soaking of pine wood chips with ionic liquids for reduced energy input during grinding, Agnieszka Brandt, James K. Erickson, Jason P. Hallett, Richard J. Murphy, Antje Potthast, Michael J. Ray, Thomas Rosenau, Michael Schrems and Tom Welton, Green Chem., 2012, DOI: 10.1039/C2GC15663F
You may also be interested in these related articles which are also free to access for 2 weeks:
Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid–water mixtures, Agnieszka Brandt, Michael J. Ray, Trang Q. To, David J. Leak, Richard J. Murphy and Tom Welton, Green Chem., 2011, 13, 2489-2499
The effect of the ionic liquid anion in the pretreatment of pine wood chips, Agnieszka Brandt, Jason P. Hallett, David J. Leak, Richard J. Murphy and Tom Welton, Green Chem., 2010, 12, 672-679
Stay up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts.
Comments Off on Soaking of pine wood chips with ionic liquids for reduced energy input during grinding