Kylie L. Luska
Institute for Technical and Macromolecular Chemistry, RWTH Aachen University, Worringerweg 1, 52074 Aachen, Germany.
The 7th Green Solvents Conference took place in the Saxonian capital city of Dresden, Germany from October 19-22, 2014. This biennial Dechema conference brings together chemists and engineers from both academia and industry to discuss their latest research discoveries and future perspectives on the fundamental aspects and application of advanced fluids. An important aspect of the Green Solvents Conference series is the selection of a unique locale, in which previous meetings have been held in the lower Rhein Valley (Bruchsal), Lake Constance (Friedrichshafen), the Bavarian Alps (Berchtesgaden) and the middle Rhein Valley (Boppard). The latest edition of the Green Solvents Conference took place in Dresden; a city renowned for its magnificent baroque architecture and role in the reunification of Germany through the “Peaceful Revolution”.
The four-day conference provided an opportunity to discuss the scientific progress and application of such alternative solvents as water, ionic liquids, supercritical fluids, green organic solvents and controllable multiphase media. The conference presentations highlighted the wide variety of chemical syntheses and processes utilizing advanced fluids including: biomass conversion, supported ionic liquid phases and continuous flow processes. A common discussion point during the conference involved various life cycle characteristics of alternative solvents such as large-scale production, toxicity and end-of-life disposal. It was emphasized that such factors need to be considered during the early stages of advanced fluid development to ensure novel chemical syntheses or processes will have a reduced environmental impact compared to conventional methods.
On Sunday evening, the conference began with the awarding of the “Willi Keim Prize” to Roberto Rinaldi from the Max-Planck-Institut für Kohlenforschung. The section of “Advanced Fluids” of Dechema established this award in 2012 to recognize an outstanding young scientist working in the field of advanced fluids and was named in honour of Prof. emeritus Wilhelm Keim (RWTH Aachen University). Dr. Keim’s research investigated the use of alternative solvents in numerous catalytic processes, in which his work was important in the development of the SHOP process (Shell Higher Olefin Process) by demonstrating and implementing the principle of biphasic catalysis for the first time on an industrial scale. Dr. Rinaldi was the recipient of the 2014 Willi Keim Award for his work on the use of alternative solvents in the conversion of biomass. His intriguing talk outlined different aqueous, ionic liquid and solvent-free (mechanochemical) methods for the processing of biomass feedstocks that have been developed within his lab.

Willi Keim Prize winner Dr. Roberto Rinaldi
The Sunday evening program also included a keynote lecture from one of the pioneers in the field of advanced fluids, Martyn Poliakoff from the University of Nottingham. His research examines the development of continuous catalytic processes using scCO2 as a mobile phase. His lecture illustrated how supercritical carbon dioxide (scCO2), an “old” solvent, can be utilized in the development of novel chemical processes. As examples, he showcased how his lab has created a self-optimizing continuous flow reactor for the conversion of levulinic acid to δ-valerolactone, a continuous method for the creation of methylmethacrylate and a continuous photocatalytic procedure toward the synthesis of artemisinin.
The Monday program began with Mark Shiflett from Dupont Central Research and Development who outlined his work toward the implementation of ionic liquids within the chemical industry. His work examines the phase behaviour of hydrofluorocarbons in imidazolium ionic liquids, in which he stressed the importance in obtaining high quality data in order to properly understand the fundamental properties of advanced fluids. He illustrated how ionic liquids have potential application within Dupont as media for the purification of tetrafluoroethylene and for the separation of hydrofluorocarbon isomers and diastereomers.
Andreas Kirschning from the Leibniz Universität Hannover presented his work on the construction of continuous flow reactors in organic synthesis. He illustrated how consecutive reactions can be carried out in continuous flow to achieved complex organic transformations. He emphasized how this approach mimics biosynthetic routes, which are not necessarily “step-economic” but are highly efficient syntheses due to the continuous processing of reaction intermediates. His lab also investigates the use of inductive heating in continuous flow processes, which involves the application of an electromagnetic field to a tubular reactor constructed of a conductive metal or filled with magnetic nanoparticles. Inductive heating allows for the creation of very high reaction temperatures and has been applied in his work for such reactions as the Claisen rearrangement and alcohol oxidation.
François Jerome from the Université de Poitiers presented his work toward the application of deep eutectic solvents in the conversion of biomass resources. Deep eutectic solvents based on such materials as choline chloride represent inexpensive, non-toxic and environmentally benign ionic liquids. His work has explored the use of deep eutectic solvents in the dissolution of crystalline cellulose and for the catalytic transformation of various biomass-derived substrates. His research has shown that deep eutectic solvents can be created by mixing choline chloride directly with biomass substrates and thus avoid the requirement of a second component to form an ionic liquid phase.
Tuesday morning began with a lecture from Fabrice Gallou from Novartis who presented his perspective on the use of alternative solvents in the pharmaceutical industry. He outlined how the driving force toward the implementation of more benign solvents is mainly dependent on its performance characteristics (e.g., reaction yield, product purity) but is also related to legislation changes and literature precedence. He emphasized that the application of alternative solvents in the pharmaceutical industry is not a matter of “if” but “when” this switch will occur. He illustrated several examples of how such solvents as N-methylpyrrolidone (NMP) and dichloromethane (DCM) are being replaced with safer solvents. Furthermore, he showed how Novartis has investigated the use of surfactants to enable various organic transformations to take place in aqueous solution.
Phillip Jessop from Queen’s University outlined his research into the development of switchable solvents. He emphasized that “green solvents” are those that impart the lowest environmental impact on an entire chemical process, in which solvent separation has one of most significant influences on the efficiency of a procedure. He highlighted his work on the creation and application of switchable hydrophilicity solvents and switchable water through the use of nitrogen-based additives and CO2. The switchability of these solvents, triggered by the application or removal of CO2, allows for facile product separations in a wide variety of processes including the production of latex, extraction of soybean oil or desalination of seawater. He also showed how his group is preparing an in silico screening method to identify the most appropriate nitrogen-based additives for the formation of switchable solvents, which combines together various performance characteristics and toxicity considerations.
The last day of the conference included a keynote lecture from David Bergbreiter from Texas A&M University who presented how “older solvents” based on oligomer hydrocarbons can accomplish “new tricks”. His work investigates the use of polyisobutylene, polyethylene and poly(N-alkylacrylamide) oligomers as recyclable solvents for catalytic reactions. Functionalization of homogeneous catalysts with polymer units imparted catalyst solubility and retention in these polymeric solvents. The thermomorphic behaviour of these polymeric solvents and catalysts allow for facile product or catalyst separation by which temperature can be used as a trigger to switch between a mono- and biphasic solvent system or a soluble and insoluble catalyst.
Phillip Savage of Pennsylvania State University presented his work on the valorization of algal biomass using aqueous phase reforming. He illustrated the advantages of converting algal biomass in comparison to lignocellulose resources (e.g., faster growth rates, avoid land usage, lignin-free feedstock) and outlined various strategies in which aqueous phase reforming of algae can be used in the production of biofuels. He also presented work within his group toward the formation of novel heterogeneous catalysts that operate under aqueous phase reforming conditions.

Poster Award Winners Jose I. Garcia, Manuela Facchin and Emilia Streng (receiving the award for Zacharias Amara)
The high quality of the oral and poster program presented at the 7th Green Solvents Conference provided a stimulating environment for the conference participants to engage and discuss the future challenges and applications of advanced fluids. Congratulations to the winners of the poster awards: Jorge I. Garcia (University of San Jorge), Manuela Facchin (Università Cà Foscari Venezia) and Zacharias Amara (University of Nottingham). The conference also provided a lively social program, which provided additional opportunities for the attendees to network and exchange ideas. The conference dinner was held at Festungsmauern am Brühlschen Garten, a baroque barrel vault dining hall located below the old city walls of Dresden, which offered a beautiful setting for the participants to enjoy some local food, drinks and entertainment.

Dr. Walter Leitner welcoming guests to the conference dinner
Academic and industrial researchers interested in the fundamental study and application of advanced fluids are encouraged to take part in the 8th Green Solvents Conference on October 16-19, 2016. In accordance with conference tradition, participants can expect the conference to be held in another inspiring city located near large amounts of the greenest solvent, water!
Comments Off on 7th Green Solvents Conference, Dresden, Germany


















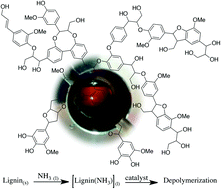
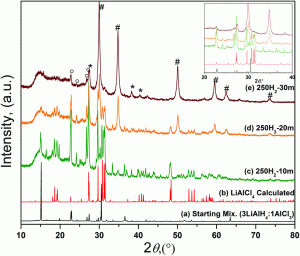
 Scientists in Canada and China have shown that the
Scientists in Canada and China have shown that the