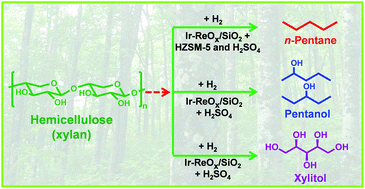
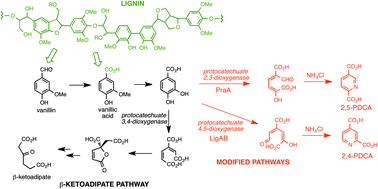
First, rhenium was introduced into an iridium impregnated silica. The resulting catalyst was capable of producing xylitol from xylan (79% yield) if used in combination with sulphuric acid in an aqueous reaction. Some success in achieving a partial reduction to pentanol was then observed when an organic solvent was introduced to the reaction. Finally, full hydrogenolysis to give pentane (in 70% yield) was accomplished by adding a HZSM-5 zeolite to the biphasic reaction.
The heterogeneous catalyst is recoverable and can be reactivated to continue providing a good yield of pentane. Thus a new flexibility to woody biomass processing has been demonstrated, complementing the more prevalent studies on cellulose and lignin.
This work is included in a online collection showcasing work presented at the 3rd International Symposium on Green Chemistry held in La Rochelle, France on 3rd-7th May 2015. Access the full collection of articles here.
Selective transformation of hemicellulose (xylan) into n-pentane, pentanols or xylitol over a rhenium-modified iridium catalyst combined with acids
Sibao Liu, Yasuyo Okuyama, Masazumi Tamura, Yoshinao Nakagawa, Akio Imai and Keiichi Tomishige
Green Chem., 2016, Advance Article. DOI: 10.1039/C5GC02183A











 High purity silicon is essential for manufacturing solar panels. Unfortunately this prerequisite conversion of silica to elemental silicon requires a lot of energy, and the associated greenhouse gas emissions are significant. It has now been demonstrated that the ashes from burning biomass (rice hulls in this case) can provide a rich source of silica than can be reduced to give solar grade silicon.
High purity silicon is essential for manufacturing solar panels. Unfortunately this prerequisite conversion of silica to elemental silicon requires a lot of energy, and the associated greenhouse gas emissions are significant. It has now been demonstrated that the ashes from burning biomass (rice hulls in this case) can provide a rich source of silica than can be reduced to give solar grade silicon. The
The 
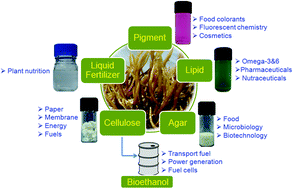 The land use issues associated with biomass production points to marine biomass as a promising alternative. Seaweed is a rich resource, abundant in the world’s oceans. In addition to its potential for biofuel production, it is also important to obtain a stream of renewable chemicals from any seaweed bio-refinery to create an
The land use issues associated with biomass production points to marine biomass as a promising alternative. Seaweed is a rich resource, abundant in the world’s oceans. In addition to its potential for biofuel production, it is also important to obtain a stream of renewable chemicals from any seaweed bio-refinery to create an 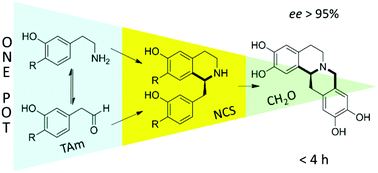 Open access communication: One-pot triangular chemoenzymatic cascades for the synthesis of chiral alkaloids from dopamine
Open access communication: One-pot triangular chemoenzymatic cascades for the synthesis of chiral alkaloids from dopamine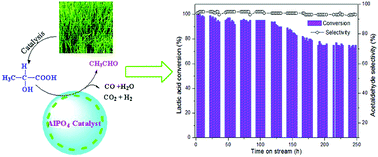 Efficient and selective conversion of lactic acid into acetaldehyde using a mesoporous aluminum phosphate catalyst
Efficient and selective conversion of lactic acid into acetaldehyde using a mesoporous aluminum phosphate catalyst