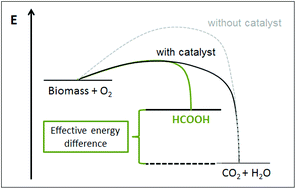
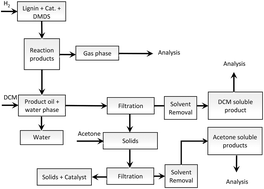
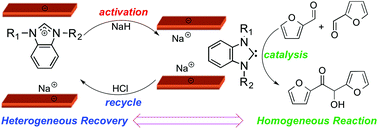
The following HOT articles have been highlighted by the reviewers of the articles as being particularly interesting or significant pieces of research. These are all free to access until 30/11/2015. The order they appear in the list has no meaning or ranking.
Zinc(II)-catalyzed reactions of carbon dioxide and propargylic alcohols to carbonates at room temperature
Jiayin Hu, Jun Ma, Qinggong Zhu, Qingli Qian, Hongling Han, Qingqing Mei and Buxing Han
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01870F, Communication
Synthesis of a polyisobutylene-tagged fac-Ir(ppy)3 complex and its application as recyclable visible-light photocatalyst in a continuous flow process
Daniel Rackl, Peter Kreitmeier and Oliver Reiser
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01792K, Paper
Precise oxygen scission of lignin derived aryl ethers to quantitatively produce aromatic hydrocarbons in water
Zhicheng Luo, Yimeng Wang, Mingyuan He and Chen Zhao
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01790D, Paper
Selective hydrogenation of levulinic acid to 1,4-pentanediol in water using a hydroxyapatite-supported Pt–Mo bimetallic catalyst
T. Mizugaki, Y. Nagatsu, K. Togo, Z. Maeno, T. Mitsudome, K. Jitsukawa and K. Kaneda
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01878A, Communication
Oxidative desulfurization of DBT with H2O2 catalysed by TiO2/porous glass
C. Shen, Y. J. Wang, J. H. Xu and G. S. Luo
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01653C, Paper
Cooperative catalysis of Pt/C and acid resin for the production of 2,5-dimethyltetrahydrofuran from biomass derived 2,5-hexanedione under mild conditions
Huacong Zhou, Jinliang Song, Qinglei Meng, Zhenhong He, Zhiwei Jiang, Baowen Zhou, Huizhen Liu and Buxing Han
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01741F, Paper
Supported nano-gold-catalyzed N-formylation of amines with paraformaldehyde in water under ambient conditions
Zhengang Ke, Yan Zhang, Xinjiang Cui and Feng Shi
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01992C, Paper
Efficient pyrido[1,2-a]benzimidazole formation from 2-aminopyridines and cyclohexanones under metal-free conditions
Yanjun Xie, Jun Wu, Xingzong Che, Ya Chen, Huawen Huang and Guo-Jun Deng
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01978H, Communication
Antimony recovery from the halophosphate fraction in lamp phosphor waste: a zero-waste approach
David Dupont and Koen Binnemans
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01746G, Paper
An efficient and recyclable tetraoxo-coordinated zinc catalyst for the cycloaddition of epoxides with carbon dioxide at atmospheric pressure
Ran Ma, Liang-Nian He and Yue-Biao Zhou
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01826A, Paper
Production of solubilized carbohydrate from cellulose using non-catalytic, supercritical depolymerization in polar aprotic solvents
Arpa Ghosh, Robert C. Brown and Xianglan Bai
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC02071A, Paper
Attractive aerobic access to the α,β-unsaturated acyl azolium intermediate: oxidative NHC catalysis via multistep electron transfer
L. Ta, A. Axelsson and H. Sundén
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01965F, Communication
Catalyst-free dehydrative SN1-type reaction of indolyl alcohols with diverse nucleophiles “on water”
Jian Xiao, Hao Wen, Liang Wang, Lubin Xu, Zhihui Hao, Chang-Lun Shao and Chang-Yun Wang
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01838B, Paper
Towards sustainable fuels and chemicals through the electrochemical reduction of CO2: lessons from water electrolysis
Antonio J. Martín, Gastón O. Larrazábal and Javier Pérez-Ramírez
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01893E, Perspective
Light driven styrene epoxidation and hydrogen generation using H2O as an oxygen source in a photoelectrosynthesis cell
P. Farràs, C. Di Giovanni, J. N. Clifford, P. Garrido-Barros, E. Palomares and A. Llobet
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01589H, Paper
Synthesis of Ni-based co-catalyst functionalized W:BiVO4 nanofibers for solar water oxidation
Ki Ro Yoon, Jong Wan Ko, Doo-Young Youn, Chan Beum Park and Il-Doo Kim
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC01588J, Communication
Biomass-derived binderless fibrous carbon electrodes for ultrafast energy storage
R. Berenguer, F. J. García-Mateos, R. Ruiz-Rosas, D. Cazorla-Amorós, E. Morallón, J. Rodríguez-Mirasol and T. Cordero
Journal Article
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC02409A, Paper
Comments Off on Recent HOT articles in Green Chemistry