James Sherwood is a guest web-writer for Green Chemistry. James is a research associate in the Green Chemistry Centre of Excellence at the University of York. His interests range from the certification and application of bio-based products, to the understanding of solvent effects in organic synthesis.
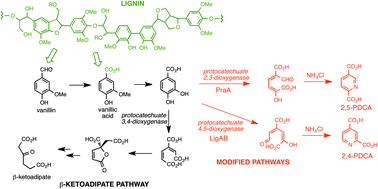 British scientists, lead by Professor Tim Bugg at the University of Warwick, have reported a new method of converting lignin into useful monomers for the chemical industry. Processing difficulties mean that lignin remains an underutilised resource for the production of renewable chemicals. Only with the development of efficient depolymerisation methods will the potential of these waste products be realised. In this latest advance, the metabolic pathways in the bacterium Rhodococcus jostii RHA1 for the degradation of lignin have been re-routed to generate aromatic dicarboxylic acids. Insertion of recombinant genes into R. jostii RHA1, followed by ammonia cyclisation generates pyridine-2,4-dicarboxylic acid or pyridine-2,5-dicarboxylic acid in yields of up to 125 mg L−1 from a wheat straw lignocellulose feed. The products have been identified as the building blocks of new bio-based polymers, and could help contribute to biomass resource efficiency and growth in the bio-polymer market.
British scientists, lead by Professor Tim Bugg at the University of Warwick, have reported a new method of converting lignin into useful monomers for the chemical industry. Processing difficulties mean that lignin remains an underutilised resource for the production of renewable chemicals. Only with the development of efficient depolymerisation methods will the potential of these waste products be realised. In this latest advance, the metabolic pathways in the bacterium Rhodococcus jostii RHA1 for the degradation of lignin have been re-routed to generate aromatic dicarboxylic acids. Insertion of recombinant genes into R. jostii RHA1, followed by ammonia cyclisation generates pyridine-2,4-dicarboxylic acid or pyridine-2,5-dicarboxylic acid in yields of up to 125 mg L−1 from a wheat straw lignocellulose feed. The products have been identified as the building blocks of new bio-based polymers, and could help contribute to biomass resource efficiency and growth in the bio-polymer market.
This article is free to access untill 31st August 2015:
Biocatalytic conversion of lignin to aromatic dicarboxylic acids in Rhodococcus jostii RHA1 by re-routing aromatic degradation pathways, Z. Mycroft, M. Gomis, P. Mines, P. Law and T. D. H. Bugg, Green Chem., 2015, Advance Article. DOI: 10.1039/C5GC01347J











