Olefin metathesis is a route to the formation of new C=C bonds, and is, thus, ubiquitous in organic chemistry. Reactions of this type include cross metathesis (CM) and ring-closing metathesis (RCP), among others, where Grubbs-type ruthenium (Ru) complexes bearing N-heterocyclic carbene (NHCs) ligands are commonly used commercial catalysts. Traditionally, chlorinated and aromatic solvents, e.g., DCM and toluene, are employed; their use, however, is accompanied by health and environmental concerns. Less hazardous reaction media, including water and ionic liquids (ILs), have been previously investigated, but special reaction conditions, or catalysts that are not readily available, are required for their success.
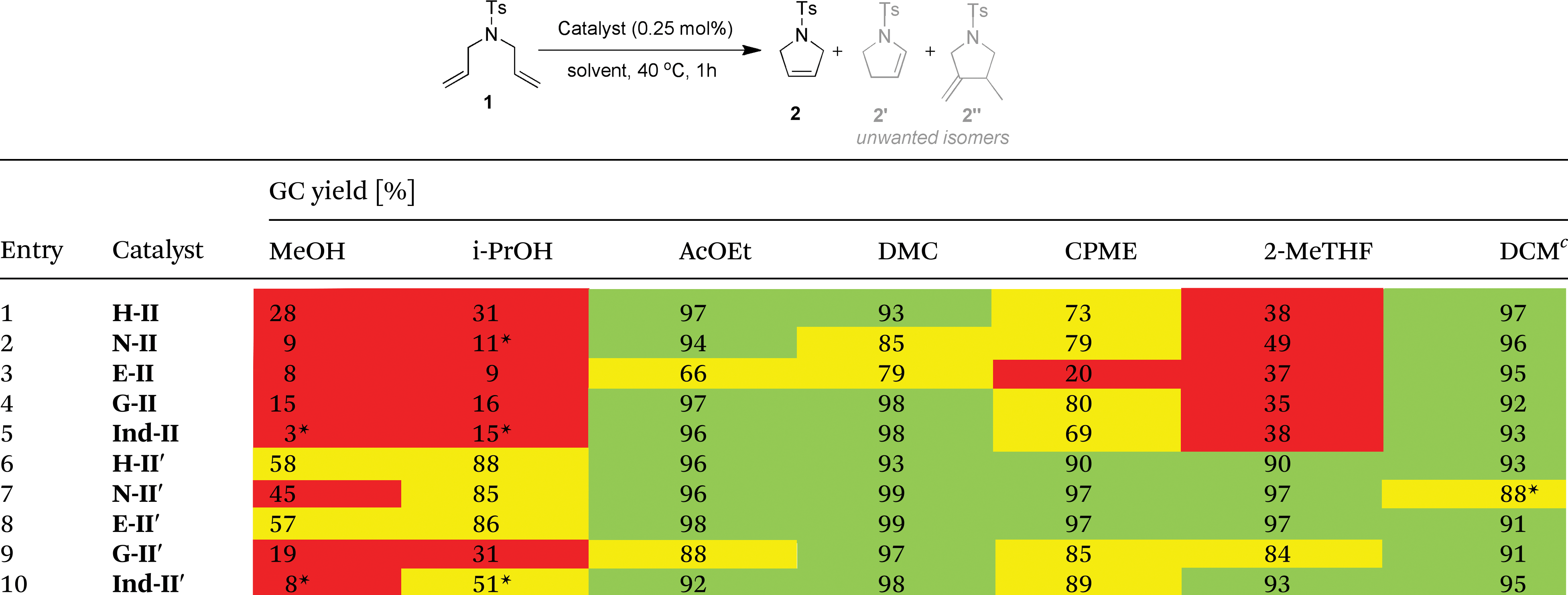 In this paper, the authors sought to evaluate the performance of several types of commercial metathesis catalysts in non-traditional solvents, viz., MeOH, i-PrOH, EtOAc, DMC, CPME, and 2-MeTHF. These solvents were chosen based on their adherence to “green” solvent selection criteria. A series of ten Ru-based catalysts with varied (NHC) ligand architecture was chosen for this study. The most effective solvent/catalyst combinations for ROP of dienes and CM of esters were first determined using model compounds. A number of substrates, having motifs prevalent in natural products, were subsequently tested in the best performing solvent, i.e., EtOAc. The results indicated that the catalyst systems, when used in ester solvents at 70 °C, produced similar yields and selectivites to analogous reactions in toluene and DCM. These results were obtained in air rather than under an inert gas. Moreover, the reactions were carried out in solvents that had not been degassed or distilled, as is typically the case. This presents a valuable alternative to the less sustainable conditions commonly used in industry and academia.
In this paper, the authors sought to evaluate the performance of several types of commercial metathesis catalysts in non-traditional solvents, viz., MeOH, i-PrOH, EtOAc, DMC, CPME, and 2-MeTHF. These solvents were chosen based on their adherence to “green” solvent selection criteria. A series of ten Ru-based catalysts with varied (NHC) ligand architecture was chosen for this study. The most effective solvent/catalyst combinations for ROP of dienes and CM of esters were first determined using model compounds. A number of substrates, having motifs prevalent in natural products, were subsequently tested in the best performing solvent, i.e., EtOAc. The results indicated that the catalyst systems, when used in ester solvents at 70 °C, produced similar yields and selectivites to analogous reactions in toluene and DCM. These results were obtained in air rather than under an inert gas. Moreover, the reactions were carried out in solvents that had not been degassed or distilled, as is typically the case. This presents a valuable alternative to the less sustainable conditions commonly used in industry and academia.
By Jenna Flogeras
To read the full article, please click the link below. The paper is free to access for 4 weeks!
In an attempt to provide green solvent selection guide for olefin metathesis, Tomasz Krzysztof Olszewski, Krzysztof Skowerski, Andrzej Tracz and Jacek Bialecki, Green Chem., 2013, DOI: 10.1039/C3GC41943F














 The front cover this month (pictured left) features work by Wouter De Soute and co-workers from Ghent, Belgium. In their work, they show that shifting from batch to continuous pharmaceutical tablet manufacturing results in a significant reduction in natural resource extraction.
The front cover this month (pictured left) features work by Wouter De Soute and co-workers from Ghent, Belgium. In their work, they show that shifting from batch to continuous pharmaceutical tablet manufacturing results in a significant reduction in natural resource extraction.  The inside front cover this month (pictured right) features work by Davit Zargarian and co-workers from Quebec, Canada. In their work they reveal a new one-pot method for the efficient and atom-economical synthesis of POCOP-type pincer complexes of divalent nickel that serve as pre-catalysts for various catalytic transformations.
The inside front cover this month (pictured right) features work by Davit Zargarian and co-workers from Quebec, Canada. In their work they reveal a new one-pot method for the efficient and atom-economical synthesis of POCOP-type pincer complexes of divalent nickel that serve as pre-catalysts for various catalytic transformations.
 Frank Vanhaecke
Frank Vanhaecke
 Reversible polymerization of novel monomers bearing furan and plant oil moieties: a double click exploitation of renewable resources
Reversible polymerization of novel monomers bearing furan and plant oil moieties: a double click exploitation of renewable resources
![GA[2]](https://blogs.rsc.org/gc/files/2013/10/GA2.gif)

