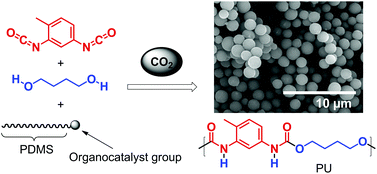
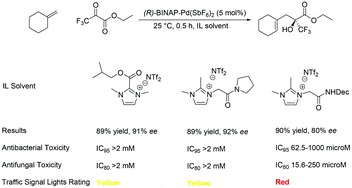
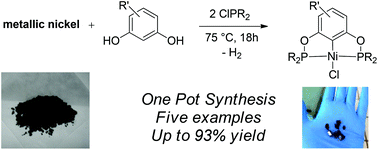
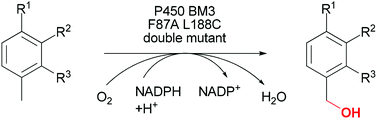
Albert Matlack, one of the founding educators of green chemistry and author of the book ‘Introduction to Green Chemistry’, selects his favourite papers in Green Chemistry this month…
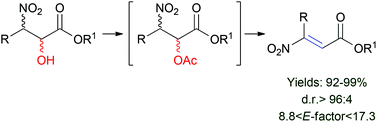
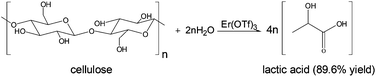
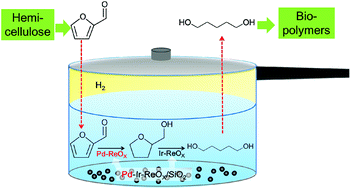
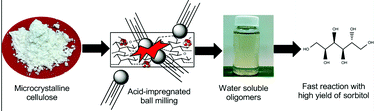 Mechanical depolymerisation of acidulated cellulose: understanding the solubility of high molecular weight oligomers, Abhijit Shrotri, Lynette Kay Lambert, Akshat Tanksale and Jorge Beltramini, Green Chem., 2013, 15, 2761–2768, DOI: 10.1039/c3gc40945g
Mechanical depolymerisation of acidulated cellulose: understanding the solubility of high molecular weight oligomers, Abhijit Shrotri, Lynette Kay Lambert, Akshat Tanksale and Jorge Beltramini, Green Chem., 2013, 15, 2761–2768, DOI: 10.1039/c3gc40945g
In this work, cellulose was milled with sulfuric acid to produce oligomers which were hydrogenated over a Ni/Pt/aluminium oxide catalyst 200 °C for an hour to produce 90% of sorbitol and mannitol. This yield is better than the efforts of others to do the job. Sorbitol can be converted to isosorbide, then to high melting point biopolymers. Further work is needed to get more than the 34.6% of the starting cellulose to dissolve.
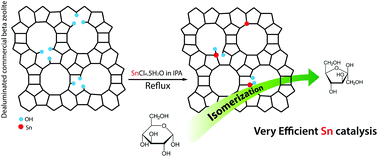
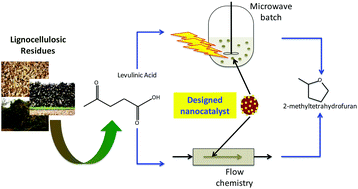 Continuous flow nanocatalysis: reaction pathways in the conversion of levulinic acid to valuable chemicals, Jose M. Bermudez, J. Angel Menéndez, Antonio A. Romero, Elena Serrano, Javier Garcia-Martinez and Rafael Luque, Green Chem., 2013, 15, 2786–2792, DOI: 10.1039/c3gc41022f
Continuous flow nanocatalysis: reaction pathways in the conversion of levulinic acid to valuable chemicals, Jose M. Bermudez, J. Angel Menéndez, Antonio A. Romero, Elena Serrano, Javier Garcia-Martinez and Rafael Luque, Green Chem., 2013, 15, 2786–2792, DOI: 10.1039/c3gc41022f
This work shows the advantages of a continuous flow system to produce biomass platform chemicals. The authors use a ThalesNano H-Cube of levulinic acid to produce 60% methyltetrohydrofuran and 40% 1,4-pentanediol in one minute at 150 °C, which shows the value of the system over a batch method.
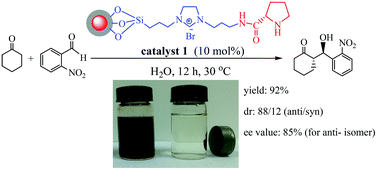
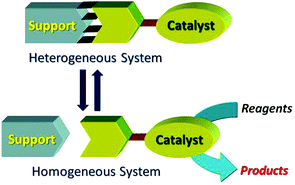
“Release and catch” catalytic systems, Michelangelo Gruttadauria, Francesco Giacalone and Renato Noto, Green Chem., 2013, 15, 2608–2618, DOI: 10.1039/c3gc41132j
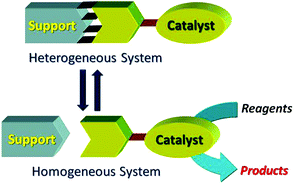 This review covers catch and release systems which do not use covalent bonding to a support. The concept is good in the sense that a homogeneous catalyst may have a single active site, whereas a heterogeneous catalyst may have several types of active sites. The systems work with a variety of metal catalysts, but activity decreases on repeating cycles. Metal leaching may be taking place. Further work is needed to make such systems practical for widespread use.
This review covers catch and release systems which do not use covalent bonding to a support. The concept is good in the sense that a homogeneous catalyst may have a single active site, whereas a heterogeneous catalyst may have several types of active sites. The systems work with a variety of metal catalysts, but activity decreases on repeating cycles. Metal leaching may be taking place. Further work is needed to make such systems practical for widespread use.











![GA[3]](https://blogs.rsc.org/gc/files/2013/09/GA3.gif)
![GA[1]](https://blogs.rsc.org/gc/files/2013/09/GA1.gif)
![GA[2]](https://blogs.rsc.org/gc/files/2013/09/GA2.gif)
 The front cover this month (pictured left) features work by Terrence Collins, Robert Tanguay and co-workers from the USA. In their Communication, they report how Zebrafish embryo developmental assays allow the relationships between catalyst structure, performance and toxicity to be mapped for seven full functional mimics of peroxidase enzymes of the TAML activator family.
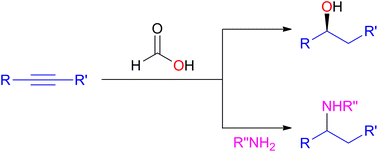
The front cover this month (pictured left) features work by Terrence Collins, Robert Tanguay and co-workers from the USA. In their Communication, they report how Zebrafish embryo developmental assays allow the relationships between catalyst structure, performance and toxicity to be mapped for seven full functional mimics of peroxidase enzymes of the TAML activator family. The inside front cover this month (pictured right) features work by Luigi Vaccaro and co-workers from Perugia, Italy. In their work they report a flow procedure which produces excellent yields of 1,2-azido alcohols and significantly minimizes waste. Furthermore, 1,2-amino alcohols are also prepared in quantitative yields.
The inside front cover this month (pictured right) features work by Luigi Vaccaro and co-workers from Perugia, Italy. In their work they report a flow procedure which produces excellent yields of 1,2-azido alcohols and significantly minimizes waste. Furthermore, 1,2-amino alcohols are also prepared in quantitative yields.