Scientists from China have developed a way to intensify photocatalytic processes, and they have utilised it for efficient photoreactor design.
Currently, photocatalysis has few commercial or industrial applications because of the complex configuration of the photoreactor that would be required, and thus difficult engineering and scale-up issues. However, Zhuhong Yang and co-workers from Nanjing University of Technology, China have developed a new photoreactor design which separates the process into two parts and has been inspired by photosynthesis in plants.
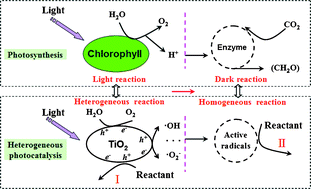
By separating the illumination and mixing parts of the photoreactor, scale-up and engineering applications will be made much easier.
For more information, please see the full article, free to read until 13th June:
Photosynthesis-inspired design approach of a liquid phase heterogeneous photoreactor, Dong Li, Kui Xiong, Kangzhong Shi, Zhuhong Yang, Chang Liu, Xin Feng and Xiaohua Lu, Green Chem., 2011, DOI: 10.1039/C1GC15082K.