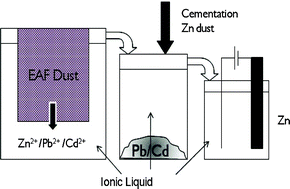
Andrew Abbott and co-workers at the University of Leicester have reviewed the use of ionic liquids for the processing of metals and metal oxides.
The processing and reprocessing of metals is possibly one of the largest energy consumers and generators of waste in the industry sector. Typically, metal extract and recovery (also known as hydrometallurgy) in solution is done in melts or very basic or acidic solutions, as metal oxides are insoluble in most molecular solvents. However, these methods have disadvantages due to their high energy demands, the amount of waste generated, and the numerous steps involved.
Ionic liquids, salts which are liquid below 100 °C, possess useful physical properties which allow them to be applied to many different reactions and processes. In this case, some ionic liquids have demonstrated much higher solubilities for metal salts than most organic solvents. Potential advantages of using ionic liquids in this field include the ability to simplify processing techniques and avoid the formation of oxide and hydroxide products during processing.
In this review Abbott and co-workers critically review the potential efficacy of ionic liquids in metal and metal oxide processing over existing methods.
This article is freely available until the 16 March 2011:
Processing of metals and metal oxides using ionic liquids, Andrew P. Abbott, Gero Frisch, Jennifer Hartley and Karl S. Ryder, Green Chem., 2011, DOI: 10.1039/C0GC00716A.