Scientists from Germany have reported a simple and robust ionic liquid-based system for the selective decomposition of formic acid to hydrogen and carbon dioxide.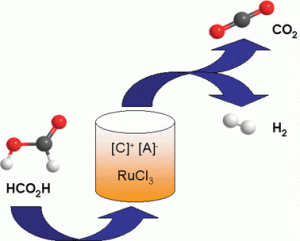
Formic acid has been proposed as an attractive hydrogen carrier substance as it can easily be stored and transported to be catalytically decomposed to release hydrogen (and CO2) on demand. Wasserscheid and his team have developed a simple and robust catalytic system involving the ionic liquid [EMMIM][OAc] and the ruthenium catalyst RuCl3 which gave excellent yields of hydrogen from formic acid. As ionic liquids have extremely low volatility, this avoids solvent contamination of the produced hydrogen stream. In addition, the system is very selective, with no carbon monoxide form during the decomposition, thereby avoiding complicated gas purification procedures later on.
However, one of the most striking features of the system reported by Wasserscheid is the recyclability of this ionic liquid-catalyst system. The RuCl3/[EMMIM][OAc] could was reused for at least another nine runs after its first use, with no decline in the selectivity or yields, illustrating the robust nature of this system. This has clear implications for further development of this process and its application in industry.
To read more please see link below for the full journal article. This article will be free to access until 2 May 2011.
M. E. M. Berger, D. Assenbaum, N. Taccardi, E. Spiecker and P. Wasserscheid, Green Chem., 2011, DOI: 10.1039/C0GC00829J,










