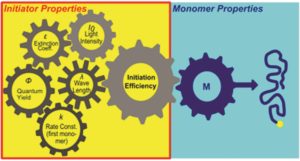
A major requirement to synthesize polymeric materials via photopolymerizations is the efficiency of the photoinitiator. This is because the amount of initiated polymer chain is crucial for the outcome of the radical polymerization. Many factors have been reported to influence this efficiency including the UV-Vis absorption properties, the irradiation wavelengths, the dissociation quantum yields and the reactivity of primary radicals towards monomers. To enable “on demand” access to a wide range of photoinitiators performance, Gescheidt and co-workers developed a tool for predicting the initiator efficiency of various type 1 (α-cleavage) photoinitiators. To achieve this, a systematic analysis of the photoinitiator performance revealed the interplay of absorption properties, dissociation quantum yields, light intensities, irradiation wavelengths and kinetics. It is important to note that important side reactions such as oxygen quenching have also been taken into account. The author’s simulations demonstrate that under ideal conditions any photoinitiator will function in an almost perfect way. However, the oxygen quenching mechanism combined with the subtleness of the photo-physical properties of the photoinitiator differentiates a well-suited photoinitiator from a less useful one. The authors conclude that a balanced combination of the intrinsic photoinitiator characteristics (i.e. absorption spectrum of initiator, quantum yields of dissociation, rate constants for primary radicals towards monomers), the major side reactions such as oxygen quenching, and the emission properties of the utilized light source (i.e. irradiation wavelengths, light intensity) is crucial to achieve the optimal initiation performance. As the authors nicely note in their conclusion, such a tool is not only useful for experienced researchers but it can also serve as an educational guide. The corresponding kinetic scheme is freely available on the author’s website and can be adjusted by any user.
Tips/comments directly from the authors:
1. A single property is not sufficient to reasonably classify a photoinitiator. A subtly interplay between absorbance, quantum yield, kinetics and the character of the light source is what matters.
2. The choice of a suitable photoinitiator strongly depends on the desired application (e.g. bulk polymerization or coatings). Here, the number of initiating radicals directs the efficiency of the polymerization and its robustness vs. oxygen inhibition.
3. The optical density of the formulation combined with the wavelength of irradiation control the thickness of the cured layer. It is crucial to be aware of the exact properties of the used light source (emission bands, intensity), since this drastically influences the amount of generated radicals and the initiation rate.
4. The Simulations rely on experimental parameters, which are straightforwardly determined (e.g. rate constants (Polym. Chem., 2018, 9, 38–47) and quantum yields (Photochem. Photobiol. Sci., 2018, 17, 660–669)) and provide an overall picture of a complex process such as the initiation of a photo-induced polymerization.
5. We are happy to answer any questions or remarks concerning our initiation model – please contact g.gescheidt-demner@tugraz.at.
FREE to read until 24th December
Choosing the ideal photoinitiator for free radical photopolymerizations: predictions based on simulations using established data, Polym. Chem., 2018, 9, 5107-5115, DOI: 10.1039/C8PY01195H
About the web writer
 Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). In January 2019, she will join the ETH Materials Department as an Assistant Professor to establish her independent group.
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). In January 2019, she will join the ETH Materials Department as an Assistant Professor to establish her independent group.