Phosphonic acid functionalized polymers find use in a wide range of applications such as metal protection, polymer electrolyte membranes flame retardancy and dentistry. This is thanks to their unique characteristics including their acidic nature, stability, proton conductivity and metal binding ability. Vinyl phosphonic acid (VPA) in particular is a structurally simple example of such monomers which is not only affordable but also provides with a polymer (PVPA) with phosphonic acid groups that are directly attached to the backbone.
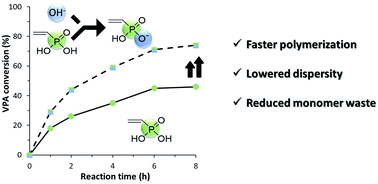
Despite the popularity and applicability of this polymer, the polymerization of VPA is typically slow yielding incomplete conversions which necessitates the need for additional costly and time-consuming purification steps. Destarac, Harrisson and co-workers were capable to circumvent this by investigating the effect of adding various alkali hydroxides to the conventional (free radical) and reversible addition-fragmentation transfer polymerization/macromolecular design by interchange of xanthates (RAFT/MADIX) radical polymerizations. Both types of polymerizations were strongly affected by the addition of NaOH. The authors found that by adding 1 equivalent of NaOH they could significantly increase the rate of the polymerization and the final conversion for both conventional and RAFT/MADIX polymerizations while larger quantities led to retardation of the reaction. A wide range of alkali hydroxides were also studied including H+, Li+, K+ and NH4+. It was shown that the dispersity of the final polymer decreases as the ionic radius of the counterion increases (H+ > Li+> Na+ > K+ > NH4+) while the acceleration of the polymerization follows the order Na+ > K+ > NH4+> Li+ > H+). Overall, the fastest rates of polymerizations were obtained in the presence of 0.5 equivalent of NaOH, while the same concentration of KOH or NH4OH allowed for a moderate acceleration on the polymerization rate combined with an improved control over the molar masses. Thus, this simple and cost-effective strategy can significantly improve the efficiency of the polymerization of VPA by simultaneously enhancing the reaction rate and the control over the molar masses.
Tips/comments directly from the authors:
- Take care to control the temperature when neutralizing the VPA – the reaction is very exothermic!
- Use NaOH for the most significant acceleration of polymerization and NH4OH for the strongest reduction in dispersity of the polymer.
- PVPA homopolymer can be precipitated in MeOH, but many PVPA-containing DHBCs must be purified by dialysis due to the small difference in solubility between PVPA and VPA and the low volatility of VPA.
Read this exciting research for free until 10/09/2017 through a registered RSC account.
Acceleration and improved control of aqueous RAFT/MADIX polymerization of vinylphosphonic acid in the presence of alkali hydroxides
Polym. Chem., 2017, 8, 3825-3832, DOI: 10.1039/c7py00747g
—————-
Dr. Athina Anastasaki is a web writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please visit this website for more