Facile synthetic and modification procedures of functionalized polymers have been the subject of extensive fundamental and applied research efforts during the last decade. The concept of ‘click’ chemistry induced a transition towards ‘on-demand’ preparation of tailored polymeric systems. The toolbox of research labs is currently loaded with a variety of established ‘click’ reactions, offering ample possibilities for macromolecular design and synthesis. Moreover, the development and valorization of novel polymer materials with a broad range of applications (medicines, electronics, bioconjugation, labeling, etc.) significantly promoted interdisciplinary research. The elaboration of innovative procedures and the combination of existing reactions in multi-step one-pot sequences further exemplify the scientific eagerness to study the possibilities and limitations of ‘click’ chemistry to the full extent.
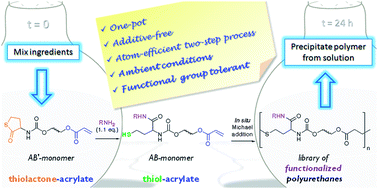
Filip Du Prez et al. have elaborated a straightforward, isocyanate-free method for the synthesis of functionalized polyurethanes, based on amine–thiol–ene conjugation. Aminolysis of a readily available AB′-urethane monomer, containing both an acrylate (A) and a thiolactone unit (B′), facilitates the preparation of various reactive thiol–acrylates. In situ polymerization via Michael addition proceeds under ambient conditions, yielding polyurethanes with a large variety of chemical functionalities. Side-chain functionality originates from the modular use of different amines, allowing for the introduction of pendent functional groups (e.g. double bond, triple bond, furfuryl, tertiary amine, morpholine) along the polyurethane backbone. Extensive model studies revealed the kinetic profile of this reaction sequence and excluded the occurrence of competing reactions, such as aza-Michael addition and disulfide formation. This mild one-pot reaction requires no additives or external trigger and the obtained polyurethanes remain soluble throughout the process, enabling post-polymerization modification in the same reaction medium.
One-pot, additive-free preparation of functionalized polyurethanes viaamine–thiol–ene conjugation by Pieter Espeel, Fabienne Goethals. Frank Driessen, Le-Thu T. Nguyen and Filip Du Prez, Polym. Chem. 2013, 4, 2449-2456.