Acetylene is widely known for its use as a fuel in welding equipment and it is also a very important building block in industrial chemical synthesis. Unfortunately, obtaining high-purity acetylene is not a trivial matter. Removing methane and carbon dioxide via cryogenic distillation is extremely energy intensive due to the low temperature required and therefore a process that avoids the need for such cooling is extremely attractive.
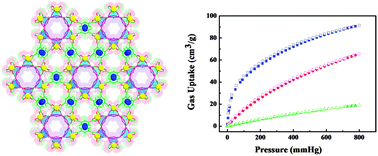
Hui Xu et al. have recently demonstrated the effective purification of acetylene at room temperature and pressure through the use of a microporous metal-organic framework, Cu6(PDC)6 . 2.6H2O (PDC = 3,4-pyridine-dicarboxylate). Known as UTSA-50, the material was designed such that its pores are not only optimally sized for gas storage but that they also contain both exposed metal atoms and pyridyl groups. This enables both electrostatic and hydrogen-bonding interactions with acetylene. The latter are thought to be the key to selectivity given the ability of pyridyl nitrogen atoms to form hydrogen bonds with acetylene but not with CO2 or CH4.
At 296 K and 1 atm the UTSA-50 framework can adsorb 91 cm-1 g-1 acetylene which is comparable to other materials with similar pore size and surface area. Henry’s law selectivities of 68.0 and 13.3 for acetylene over carbon dioxide and methane, respectively, are extremely promising. In fact, the selectivity for CO2 is, according to the authors, the highest ever reported.
J. Mater. Chem. A, 2013, 2, 77. DOI: 10.1039/c2ta00155a
James Serginson is a guest web writer for the Journal of Materials Chemistry blog. He currently works at Imperial College London carrying out research into nanocomposites.
To keep up-to-date with all the latest research, sign-up to our RSS feed or Table of contents alert.