For Green Chemistry, the top 10 most accessed articles in February were as follows:
Deconstruction of lignocellulosic biomass with ionic liquids
Agnieszka Brandt, John Gräsvik, Jason P. Hallett and Tom Welton
Green Chem., 2013, 15, 550-583
DOI: 10.1039/C2GC36364J, Critical Review
Iron-catalyzed direct alkenylation of sp3(C–H) bonds via decarboxylation of cinnamic acids under ligand-free conditions
Hailong Yang, Hong Yan, Peng Sun, Yan Zhu, Linhua Lu, Defu Liu, Guangwei Rong and Jincheng Mao
Green Chem., 2013, 15, 976-981
DOI: 10.1039/C3GC37131J, Paper
Glycerol carbonate as a versatile building block for tomorrow: synthesis, reactivity, properties and applications
Matthieu O. Sonnati, Sonia Amigoni, Elisabeth P. Taffin de Givenchy, Thierry Darmanin, Olivier Choulet and Frédéric Guittard
Green Chem., 2013, 15, 283-306
DOI: 10.1039/C2GC36525A, Critical Review
Food waste biomass: a resource for high-value chemicals
Lucie A. Pfaltzgraff, Mario De bruyn, Emma C. Cooper, Vitaly Budarin and James H. Clark
Green Chem., 2013, 15, 307-314
DOI: 10.1039/C2GC36978H, Perspective
Ohmic heating as a new efficient process for organic synthesis in water
Joana Pinto, Vera L. M. Silva, Ana M. G. Silva, Artur M. S. Silva, José C. S. Costa, Luís M. N. B. F. Santos, Roger Enes, José A. S. Cavaleiro, António A. M. O. S. Vicente and José A. C. Teixeira
Green Chem., 2013, 15, 970-975
DOI: 10.1039/C3GC36881E, Paper
Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass
David Martin Alonso, Stephanie G. Wettstein and James A. Dumesic
Green Chem., 2013, 15, 584-595
DOI: 10.1039/C3GC37065H, Critical Review
Eco-efficient, regioselective and rapid access to 4,5-disubstituted 1,2,3-thiadiazoles via [3 + 2] cycloaddition of α-enolicdithioesters with tosyl azide under solvent-free conditions
Maya Shankar Singh, Anugula Nagaraju, Girijesh Kumar Verma, Gaurav Shukla, Rajiv Kumar Verma, Abhijeet Srivastava and Keshav Raghuvanshi
Green Chem., 2013, 15, 954-962
DOI: 10.1039/C3GC37047J, Paper
Catalytic etherification of glycerol with short chain alkyl alcohols in the presence of Lewis acids
Fei Liu, Karine De Oliveira Vigier, Marc Pera-Titus, Yannick Pouilloux, Jean-Marc Clacens, Floryan Decampo and François Jérôme
Green Chem., 2013, 15, 901-909
DOI: 10.1039/C3GC36944G, Paper
Selective oxidation of alcohols and aldehydes over supported metal nanoparticles
Sara E. Davis, Matthew S. Ide and Robert J. Davis
Green Chem., 2013, 15, 17-45
DOI: 10.1039/C2GC36441G, Critical Review
Multicomponent reactions in unconventional solvents: state of the art
Yanlong Gu
Green Chem., 2012, 14, 2091-2128
DOI: 10.1039/C2GC35635J, Critical Review
Take a look at the articles, and then let us know your thoughts and comments below.
Fancy submitting your own work to Green Chemistry? You can submit online today, or email us with your ideas and suggestions.
Comments Off on Top 10 most accessed articles in February
















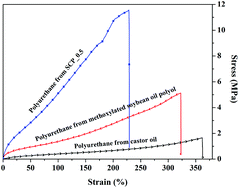
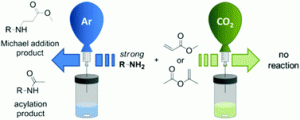
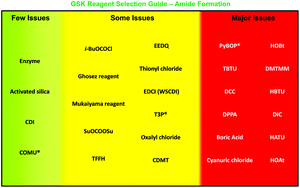
 The ACS Green Chemistry Institute®’s
The ACS Green Chemistry Institute®’s