Here are the latest HOT papers published in Green Chemistry as recommended by the referees:
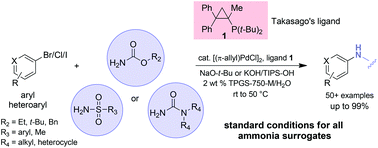
Installation of protected ammonia equivalents onto aromatic & heteroaromatic rings in water enabled by micellar catalysis
Nicholas A. Isley, Sebastian Dobarco and Bruce H. Lipshutz
Green Chem., 2014, Advance Article, DOI: 10.1039/C3GC42188K
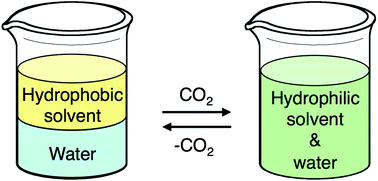
Design and evaluation of switchable-hydrophilicity solvents
Jesse R. Vanderveen, Jeremy Durelle and Philip G. Jessop
Green Chem., 2014, Advance Article, DOI: 10.1039/C3GC42164C
Cleaving C–H bonds with hyperthermal H2: facile chemistry to cross-link organic molecules under low chemical- and energy-loads
Tomas Trebicky, Patrick Crewdson, Maxim Paliy, Igor Bello, Heng-Yong Nie, Zhi Zheng, Xiaoli Fan, Jun Yang, Elizabeth R. Gillies, Changyu Tang, Hao Liu, K. W. Wong and W. M. Lau
Green Chem., 2014, Advance Article, DOI: 10.1039/C3GC41460D
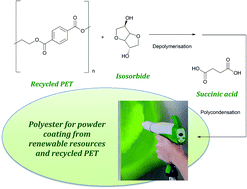
Sustainable polyesters for powder coating applications from recycled PET, isosorbide and succinic acid
C. Gioia, M. Vannini, P. Marchese, A. Minesso, R. Cavalieri, M. Colonna and A. Celli
Green Chem., 2014, Advance Article, DOI: 10.1039/C3GC42122H
All the papers listed above are free to access for the next 4 weeks!














 The front cover this month (pictured left) features work by Brian Davison and co-workers. In their work they investigate the mechanism of biomass breakdown. Understanding this process should lead to more efficient use of biomass.
The front cover this month (pictured left) features work by Brian Davison and co-workers. In their work they investigate the mechanism of biomass breakdown. Understanding this process should lead to more efficient use of biomass. The inside front cover this month (pictured right) features work by Kevin Moeller and co-workers from Missouri, USA. In their work they set up a simple solar-electrochemical reaction to recycle Os(VIII)-, TEMPO-, Ce(IV)-, Pd(II)-, Ru(VIII)-, and Mn(V)-oxidants.
The inside front cover this month (pictured right) features work by Kevin Moeller and co-workers from Missouri, USA. In their work they set up a simple solar-electrochemical reaction to recycle Os(VIII)-, TEMPO-, Ce(IV)-, Pd(II)-, Ru(VIII)-, and Mn(V)-oxidants.




