A biotechnological process to transform lignin into an adhesive opens the door on an eco-friendly strategy for recycling carpets, new research shows.
Traditional carpets consist of yarns stuck to a backing fabric by an adhesive – usually synthetic latex. As part of the production process, the latex is cured at high temperatures, but this creates a non-recyclable material as the latex is almost impossible to remove at the end of a carpet’s life. As a result, almost all carpets are disposed of by burning in an incinerator.
With a view to finding a more environmentally friendly solution to carpet disposal, Tzanko Tzanov and his team at the Polytechnic University of Catalonia in Barcelona, Spain, decided to replace the synthetic latex with an organic lignin-based adhesive to produce a renewable woollen floor covering.
Lignin is an aromatic polymer that reinforces cellulose fibres in plants and is readily available as a waste product of paper and biofuel production.
It can be easily converted into an adhesive using laccase, an enzyme found in plants and fungi. ‘Lignin is transformed by an oxidative enzymatic process that activates the phenolic structures, which can then react chemically with the wool fibres and bind them to the backing,’ explains Tzanov. The process is carried out at much lower temperatures than in latex production – around 50°C rather than 150°C – making it much more environmentally friendly.

The carpets can degrade and be recycled as a soil fertiliser
The laccase enzymes that convert lignin into an adhesive are also involved in its biodegradation, meaning that the carpets can be recycled at the end of their usable life. Instead of being incinerated, the carpets are shredded and returned to nature, where they degrade and can be used as a soil fertiliser.
Diego Moldes Moreira, an expert in natural products and bioprocesses at the University of Vigo in Spain is impressed by the innovative and sustainable solution. ‘We could expect to find the proposed biotech carpets in stores in the short–medium term,’ he says. In fact, Tzanov’s team are already working with Dutch companies, James and Best Wool Carpets, on an industrial scale-up.
You can also read this article in Chemistry World»
Read the original journal article in Green Chemistry – it’s free to access until 27th March:
An enzymatic approach to develop a lignin-based adhesive for wool floor coverings
Elisabetta Aracri, Carlos Díaz Blanco and Tzanko Tzanov
Green Chem., 2014, Accepted Manuscript, DOI: 10.1039/C4GC00063C, Paper
Comments Off on Natural adhesive brings new life to old carpets
 Shrimp shells that would otherwise be thrown away by the seafood industry have been turned into tough fibres that can harvest valuable metals from water.
Shrimp shells that would otherwise be thrown away by the seafood industry have been turned into tough fibres that can harvest valuable metals from water.










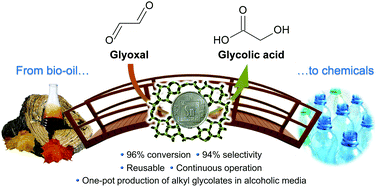
 The outside front cover this month (pictured left) features work by Javier Pérez-Ramírez and co-workers from Zurich, Switzerland. In their work they report how the Lewis-acid catalysed isomerisation of bio-oil derived glyocal over tin-based zeolites efficiently and sustainably produces glycolic acid and alykyl glycolates.
The outside front cover this month (pictured left) features work by Javier Pérez-Ramírez and co-workers from Zurich, Switzerland. In their work they report how the Lewis-acid catalysed isomerisation of bio-oil derived glyocal over tin-based zeolites efficiently and sustainably produces glycolic acid and alykyl glycolates. The inside front cover this month (pictured right) features work by Philip Jessop and co-workers from Ontario, Canada. In their work they focus on switchable-hydrophilicity solvents (SHS), which can switch reversibly between one form that is miscible with water and another that forms a biphasic mixture with water. They report new examples and compare them in terms of safety and environmental impact.
The inside front cover this month (pictured right) features work by Philip Jessop and co-workers from Ontario, Canada. In their work they focus on switchable-hydrophilicity solvents (SHS), which can switch reversibly between one form that is miscible with water and another that forms a biphasic mixture with water. They report new examples and compare them in terms of safety and environmental impact.




 The front cover this month (pictured left) features a review by Jesse Hensley and co-workers from Golden, Colorado. In their article they focus on recent model compound studies of catalysts for hydrodeoxygenation of biomass pyrolysis products, with an emphasis on mechanisms, reaction networks, and structure–function relationships.
The front cover this month (pictured left) features a review by Jesse Hensley and co-workers from Golden, Colorado. In their article they focus on recent model compound studies of catalysts for hydrodeoxygenation of biomass pyrolysis products, with an emphasis on mechanisms, reaction networks, and structure–function relationships. The inside front cover this month (pictured right) features work by Andreas Heyden and co-workers from Columbia, South Carolina. In their work they report a theoretical study of the effects of various solvents on the mechanism of the hydrodeoxygenation of propanoic acid over Pd(111).
The inside front cover this month (pictured right) features work by Andreas Heyden and co-workers from Columbia, South Carolina. In their work they report a theoretical study of the effects of various solvents on the mechanism of the hydrodeoxygenation of propanoic acid over Pd(111).