|
The 3rd International Symposium on Green Chemistry (ISGC) took place on 3rd-7th May in La Rochelle, France. This was sponsored by Green Chemistry and a number of poster prizes were awarded by Green Chemistry Advisory Board member Professor Robin Rogers of McGill University, Canada and Dr Francois Jerome, University of Poitiers, France.
The first prize was awarded to Ana Franco of the University of Cordoba in Spain for her poster ‘Waste to materials: synthesis and applications of mesoporous silicates from rice husk‘. Felix Aremando Reano, of Chaire ABI – AgroParisTech, France, received the second prize for his poster ‘Determination of antioxidant activity of new biobased macrobisphenols‘, and Clemence Queffélec, University of Nantes, France, was awarded the third prize his poster ‘Hydrothermal liquefaction as a route to transform microalgae residues in bio-asphalt‘. |
Poster Prizes at the 3rd International Symposium on Green Chemistry
A photochemical method for separating rare earth metals
Rare earth metals are notoriously hard to separate from one another, due to the similarity of their chemical properties. At present, the complex series of solvent extraction steps to extract rare earths from their ores are only carried out in China. With their increasing utilisation in modern technologies, scientists have been collaborating to develop cleaner less intensive methods of rare earth separation.
Tom Van Gerven and Koen Binnemans of the University of Leuven in Belgium have worked together to combine their expertise and develop a photochemical method for extracting the europium and yttrium from an ionic liquid solution. Both elements are present in their trivalent state, but if europium absorbs light of the correct wavelength (provided by a low pressure mercury lamp) it will reduce to the divalent state and be precipitated out.
 |
Want to know more?
Read the full article in Chemistry World by Jonathan Midgley.
Or, take a look at the original article which is free to access until 8th July 2015:
“Photochemical recycling of europium from Eu/Y mixtures in red lamp phosphor waste streams” by B Van den Bogaert et al., DOI:10.1039/c4gc02140a
Recent HOT articles in Green Chemistry
Check out the following HOT articles, these have all been made free to access for a limited time:
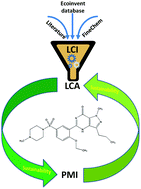
Life Cycle Inventory improvement in the pharmaceutical sector: assessment of the sustainability combining PMI and LCA tools
Daniele Cespi, Evan S. Beach, Thomas E. Swarr, Fabrizio Passarini, I. Vassura, Peter J. Dunn and Paul T. Anastas
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00424A
Conventional and microwave assisted hydrolysis of urban biowastes to added value lignin-like products
Daniele Rosso, Jiajun Fan, Enzo Montoneri, Michele Negre, James Clark and Davide Mainero
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00357A
Efficient Bromination of Olefins, Alkynes, and Ketones with Dimethyl Sulfoxide and Hydrobromic Acid
Song Song, Xinwei Li, Xiang Sun, Yizhi Yuan and Ning Jiao
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00528K
Nanoclusters of Cu (II) Supported on Nanocrystalline W (VI) Oxide: A Potential Catalyst for Single-Step Conversion of Cyclohexane to Adipic Acid
Shankha S. Acharyya, Shilpi Ghosh and Rajaram Bal
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00379B
Professor Roger A. Sheldon elected Fellow of the Royal Society
|
|
We would like to congratulate Professor Roger Sheldon, of Delft University of Technology and Green Chemistry Advisory Board member, in being elected as a fellow of the Royal Society. Fellows of the Royal Society are elected for life through a peer review process on the basis of excellence in science.
As Professor of Biocatalysis & Organic Chemistry, Roger is recognised for leading a distinguished career in which he has made pioneering contributions to catalytic oxidation, biocatalysis and green chemistry and has enabled the gap between organic synthesis and catalysis to be bridged. He also introduced the E-factor which has played a major role in drawing attention to the problem of waste generation in chemicals manufacture and provides an impetus for developing cleaner and more sustainable processes. In his most recent appointment as Distinguished Professor of Biocatalysis Engineering at the School of Chemistry at the University of the Witwatersrand, Johannesburg, Republic of South Africa, Roger will be involved in the development of green (enantio)selective biocatalytic processes. Roger is also Chief Executive Officer of CLEA Technologies B.V., a Biotech company specializing in the development of green biocatalytic processes and the cost-effective immobilization of enzymes as cross-linked enzyme aggregates. Roger is revered by the green chemistry community as one of the founding fathers of the field and of this journal. |
Read a selection of Roger’s papers – free to access until 15th June 2015:
The E Factor: fifteen years on, Roger A. Sheldon, Green Chem., 2007,9, 1273-1283
DOI: 10.1039/B713736M,
Green solvents for sustainable organic synthesis: state of the art, Roger A. Sheldon, Green Chem., 2005,7, 267-278
DOI: 10.1039/B418069K,
Biocatalysis in ionic liquids, Roger A. Sheldon, Rute Madeira Lau, Menno J. Sorgedrager, Fred van Rantwijk and Kenneth R. Seddon, Green Chem., 2002,4, 147-151
DOI: 10.1039/B110008B,
Professor Dr Roger A. Sheldon—65 years on, Ilya I. Moiseev, Shun-Ichi Murahashi, Martyn Poliakoff, Kenneth R. Seddon and Vytas K. Švedas, Green Chem., 2008,10, 270-270
DOI: 10.1039/B719347P
Richard P. Wool
 Professor Richard P. Wool, a leading figure in the green chemistry community, sadly died on 24th March 2015. Richard Wool was Professor of Chemical and Biomolecular Engineering at the University of Delaware in the United States, and headed the Affordable Composites from Renewable Sources (ACRES), which carried out work to develop uses for bio-materials such as chicken feathers and soybeans to create a diversity of products from tractors to circuit boards to a synthetic fabric named Eco-Leather.
Professor Richard P. Wool, a leading figure in the green chemistry community, sadly died on 24th March 2015. Richard Wool was Professor of Chemical and Biomolecular Engineering at the University of Delaware in the United States, and headed the Affordable Composites from Renewable Sources (ACRES), which carried out work to develop uses for bio-materials such as chicken feathers and soybeans to create a diversity of products from tractors to circuit boards to a synthetic fabric named Eco-Leather.
After completing his Bachelors degree in Chemistry in his hometown of Cork, Professor Wool moved to Utah in the United States where he completed his Masters degree and Ph.D.. This is also where he started to build his illustrious career that focussed on improving materials synthesis in order to reduce the impact this may have on the environment and on human health. He received a number of accolades for his contribution to green chemistry, including the ACS Award for Affordable Green Chemistry, the U.S.A EPA’s Presidential Green Chemistry Challenge Award for his work in Sustainable Polymers and Composites and he became a Fellow of the Royal Society of Chemistry in January 2015.
Professor Wool was also a member of the Green Chemistry Advisory Board and his contribution to the journal and the community will be sincerely missed. Green Chemistry would like to send our deepest condolences to Richard Wool’s family and friends.
Credit: University of Delaware
Call for Posters – 3rd International Conference of the Cluster of Excellence “Tailor-Made Fuels from Biomass”
Be part of the 3rd International Conference of the Cluster of Excellence “Tailor-Made Fuels from Biomass”! This conference is open to participants from science and industry interested in fields related to biomass and biofuels. The following topics will be addressed in separate sessions during the conference:
- Biomass Fractionation and Pre-treatment
- Enzymatic and Catalytic Biomass Processing
- Catalytic Synthesis and Conversion of Biomass-based Streams to Platform Molecules and Fuels
- (Bio-)refinery Process Optimization
- Injection, Ignition and Combustion of Biofuels
- Combustion Process and Exhaust Gas Aftertreatment Optimization of Biofuels
Recent HOT GC Articles
Check out the following HOT articles, these have all been made free to access for a limited time:
Recycling of rare earths from NdFeB magnets using a combined leaching/extraction system based on the acidity and thermomorphism of the ionic liquid [Hbet][Tf2N]
David Dupont and Koen Binnemans
Green Chem., 2015,17, 2150-2163
DOI: 10.1039/C5GC00155B
Upgrading biogenic furans: blended C10–C12 platform chemicals via lyase-catalyzed carboligations and formation of novel C12 – choline chloride-based deep-eutectic-solvents 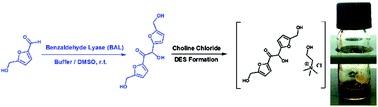
Joseph Donnelly, Christoph R. Müller, Lotte Wiermans, Christopher J. Chuck and Pablo Domínguez de María
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00342C
From simple organobromides or olefins to highly value-added bromohydrins: a versatile performance of dimethyl sulfoxide
Song Song, Xiaoqiang Huang, Yu-Feng Liang, Conghui Tang, Xinwei Lia and Ning Jiao
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00184F
Extraction and separation of neodymium and dysprosium from used NdFeB magnets: an application of ionic liquids in solvent extraction towards the recycling of magnets
Sofía Riaño and Koen Binnemans
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00230C, Paper
Greening the global phosphorus cycle: how green chemistry can help achieve planetary P sustainability
Paul J. A. Withers, James J. Elser, Julian Hilton, Hisao Ohtake, Willem J. Schipper and Kimo C. van Dijk
Green Chem., 2015,17, 2087-2099
DOI: 10.1039/C4GC02445A, Perspective
Stimuli-responsive/rheoreversible hydraulic fracturing fluids as a greener alternative to support geothermal and fossil energy production 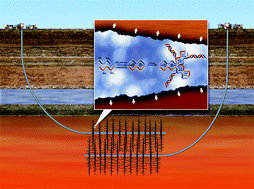
H. B. Jung, K. C. Carroll, S. Kabilan, D. J. Heldebrant, D. Hoyt, L. Zhong, T. Varga, S. Stephens, L. Adams, A. Bonneville, A. Kuprat and C. A. Fernandez
Green Chem., 2015, Advance Article
DOI: 10.1039/C4GC01917B, Paper
Fluorine gas for life science syntheses: green metrics to assess selective direct fluorination for the synthesis of 2-fluoromalonate esters
Antal Harsanyi and Graham Sandford
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00402K, Paper
Layered MoS2 nanoparticles on TiO2 nanotubes by a photocatalytic strategy for use as high-performance electrocatalysts in hydrogen evolution reactions
Chenhui Meng, Zhaoyue Liu, Tierui Zhang and Jin Zhai
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00272A, Communication
Ionic liquid-stabilized nanoparticles as catalysts for the conversion of biomass
K. L. Luska, P. Migowski and W. Leitner
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00231A, Critical Review
Green Chemistry themed issue on Elemental Recovery and Sustainability now online
Issue 4 of Green Chemistry is a part themed issue on ‘Elemental Recovery and Sustainability‘ focusing on how we can develop methods to ensure that elements are available for use by future generations through sustainable use and recovery.
The guest editors for this themed collection are James Clark (University of York, UK), Andrew Hunt (University of York, UK), Avtar Matharu (University of York, UK) and Alex King (Ames Labs, USA), read their editorial for free here.
 The outside front cover of the issue features the Critical Review “Bio-derived materials as a green route for precious & critical metal recovery and re-use” by Jennifer R. Dodson, Helen L. Parker, Andrea Muñoz García, Alexandra Hicken, Kaana Asemave, Thomas J. Farmer, He He, James H. Clark and Andrew J. Hunt. In this article they give an overview of research in critical and precious metal recovery using biosorption, application to real-life wastes and uses of the metal-loaded materials.
The outside front cover of the issue features the Critical Review “Bio-derived materials as a green route for precious & critical metal recovery and re-use” by Jennifer R. Dodson, Helen L. Parker, Andrea Muñoz García, Alexandra Hicken, Kaana Asemave, Thomas J. Farmer, He He, James H. Clark and Andrew J. Hunt. In this article they give an overview of research in critical and precious metal recovery using biosorption, application to real-life wastes and uses of the metal-loaded materials.
The inside front cover of the issue features the Paper “Recycling of rare earths from NdFeB magnets using a combined leaching/extraction system based on the acidity and thermomorphism of the ionic liquid [Hbet][Tf2N]” by David Duponta and Koen Binnemans. In this article they describe how a new recycling process was developed to recover rare earths from roasted NdFeB magnets using the thermomorphic and acidic properties of the ionic liquid [Hbet][Tf2N] to achieve a combined leaching/extraction system.
These two articles are free to access until 15th May and there are also a number of open access articles within the issue:
Recent publication in Green Chemistry on fracking – Open Forum
Green Chemistry has recently published an article on the topic of fracking entitled ‘Stimuli-responsive/rheoreversible hydraulic fracturing fluids as a greener alternative to support geothermal and fossil energy production’. This manuscript caused some debate at the Editorial Office in Cambridge due to the controversial nature of fracking and, more specifically, the validity of publishing an article on this topic in Green Chemistry.
After examination by a number of reviewers, the Editorial Office and the Chair of the Editorial Board, Professor Walter Leitner, it was decided that the manuscript was suitable for publication in Green Chemistry. However, given the controversy surrounding this topic we felt the article should be accompanied by an Editorial explaining to the community why we chose to publish and offering an avenue for debate and discussion via the online comments section on this blog.
Professor Leitner has prepared an Editorial that can be viewed below and we invite an open discussion via the comments thread of this blog. We welcome any comments to be made or opinions voiced.
If you would like to contact the Editorial Office directly you can do so by emailing green-rsc@rsc.org.
The subject of ‘fracking’ in Green Chemistry
Chair of the Editorial Board Walter Leitner discusses the subject of ‘fracking’ in Green Chemistry.
Dear Readers,
On 2nd October 2014, we received a manuscript entitled “Stimuli-Responsive/Rheoreversible Hydraulic Fracturing Fluids as an Alternative to Support Geothermal and Fossil Energy Production” at the Cambridge office. Upon careful examination of its content, we had a very serious discussion at the Editorial Office on whether the paper would fall within the scope of Green Chemistry and should be sent out for review. In the end, we came to the conclusion that we wanted to have the scientific quality examined through the review process and to gather the opinions of those reviewers on whether the work was in keeping with the Principles of Green Chemistry. Three referees suggested acceptance with some revisions, and you can find the final result published in this issue (DOI: 10.1039/C4GC01917B).
It is highly unusual to comment on a single paper and in particular on the reviewing process in an Editorial. Let me try to explain the reasons for this, which are closely related to our initial (and still existing) dilemma. Fracking is a very controversial technique for which a number of potential hazards to the environment are discussed. Most significantly, one may raise the fundamental question of whether an increased exploitation of fossil resources is inherently incompatible with the Principles of Green Chemistry. To be honest, we were unable to find a consensus and a final answer to this question ourselves. Thus, we turned to the classical maxim “in dubio pro reo” (Lat.: when in doubt, for the accused) and decided to evaluate primarily the scientific and technological aspects of the work.
When asked about the relationship between Sustainable Development and Green Chemistry, I have often used the phrase “If Sustainability is your goal, Green Chemistry is the way”. Is unconventional oil and gas recovery a sustainable technology? Certainly not in the long term – but it is and will be for quite some time influencing the basis of our raw material and energetic value chain, having an enormous impact on many aspects of our environment today. “That’s a fact. It’s a thing we can’t deny” (Katie Melua, Nine Million Bicycles). Thus, we cannot close our eyes and ignore the environmental problems that are associated with this technology. One of them is the use of the fracking fluid and all reviewers agreed that the authors of the study have carried out a sound and in depth study and presented data that might help to lower the impact of this particular aspect of the fracking industry. They have addressed this important issue following a molecular design approach in line with green chemistry principles.
Does this mean that we will now all at a sudden encourage the green chemistry community to focus on improvements of existing technologies, even if they are only incremental steps aside into the right direction on otherwise clearly unsustainable paths? Certainly not: we need to continue our efforts to contribute with fundamentally new approaches to a sustainable chemical industry and lay the basis for disruptive green technologies. However, we also recognize that things are not always black or white and there are more than 50 shades of green. In this particular case, we have decided to bring the topic on the table and to shed some light on the chemistry that is involved in fracking from the green chemistry perspective. We would be very much interested to get your feedback on this decision and have opened a discussion forum on the Green Chemistry blog.
I wish you a stimulating reading, not just with this article, but of course also with the many other fascinating examples of scientific creativity and dedicated research efforts in the issues of the Green Chemistry journal.
Walter Leitner
Ionic liquid a perfect fit for rare earth recycling
Jonathan Midgley writes about a hot Green Chemistry article for Chemistry World
Chemists in Belgium have shown how an intriguing ionic liquid they developed 10 years ago can recover valuable rare earth metals from stockpiles of used fluorescent lamps and magnets.
Rare earth metals are important in high tech applications, but China controls most of the world’s dwindling supply, periodically setting export quotas and driving up prices. They occur naturally elsewhere, but new production is time consuming and costly to establish.

- It is estimated that by 2020 stockpiled lamp phosphor waste will contain around 25,000 tons of rare earths
Read the full article in Chemistry World»
Read the journal articles in Green Chemistry:
Rare-earth recycling using a functionalized ionic liquid for the selective dissolution and revalorization of Y2O3:Eu3+ from lamp phosphor waste
David Dupont and Koen Binnemans
Journal Article
Green Chem., 2015,17, 856-868
DOI: 10.1039/C4GC02107J, Paper
Open Access
Recycling of rare earths from NdFeB magnets using a combined leaching/extraction system based on the acidity and thermomorphism of the ionic liquid [Hbet][Tf2N]
David Dupont and Koen Binnemans
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00155B, Paper
Free to access until 7 May 2015