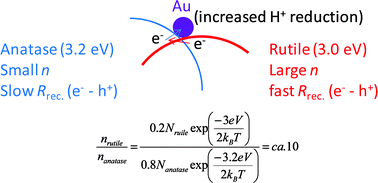
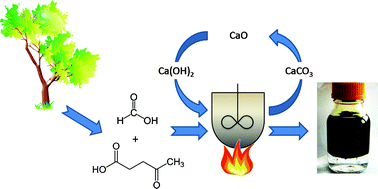
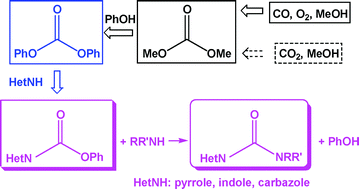
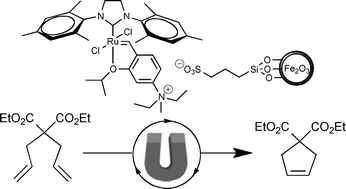
The Green Chemistry Centre in conjunction with the CIA (Chemical Industries Association) is organising the 2nd MUSC (Manufacturing Using Sustainable Chemistry) event, which will take place on Thursday 16th February 2012 at the Innovation Centre, York Science Park.
The event will be centred around Microwave-enhanced chemistry and the alternative uses of microwave heating. It will coincide with the opening of the Biorenewables Development Centre (BDC) on the York Science Park. The biorefinery R&D facility will be unique to the UK and will showcase state-of-the-art microwave-biomass processors and other clean chemical and white biotechnology with open-access to academic and industrial collaborators. The programme for the day will consist of presentations, poster sessions and a tour of the BDC. Speakers will include Mike Lancaster of the CIA, Dr Adrian Higson of the NNFCC and Dr Marilena Radoiu of Sairem.
Please see this web link to the flyer and registration form: http://www.musc-network.co.uk/flyer%20and%20form.pdf
You may also be interested in Starbons® Day at the Green Chemistry Centre on the 16th July 2012 – please see the flyer for further details: http://www.greenchemistrynetwork.org/pdf/starbons_day_flyer.pdf











 Anett Schallmey and Bruno Bühler,
Anett Schallmey and Bruno Bühler, 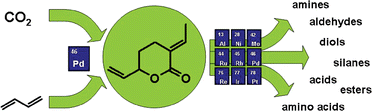 Use of carbon dioxide in chemical syntheses via a lactone intermediate
Use of carbon dioxide in chemical syntheses via a lactone intermediate