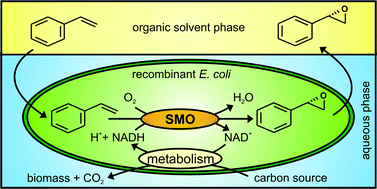
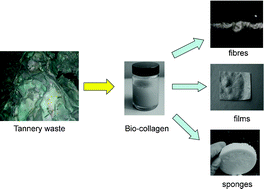
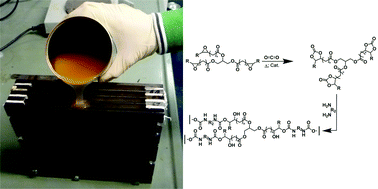
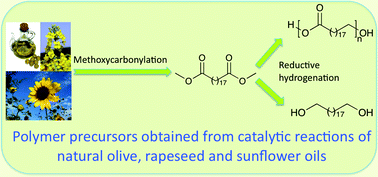
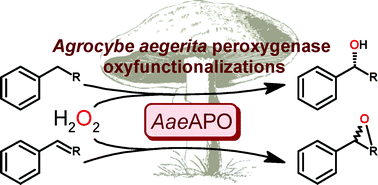
Catalysis is one of the most important areas of process and synthetic chemistry. In view of the vast range of applications, there has been extensive research into making catalysts and catalytic reactions more economical and environmentally friendly. One aspect of this is developing catalysts which can be recycled and reused over and over again with minimal effort. This can be particularly important when considering the viability of a process for industrial development, as catalysts are often expensive and so need to be recovered wherever possible in order to keep down costs.
Green Chemistry provides an excellent forum for such work and below is a selection of the cutting edge research we have published in this area over the last couple of years. These articles have been made free to access until the 27th February 2012, so make the most of this and take a look…
Why not keep up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts!
Heterogeneously catalysed Strecker-type reactions using supported Co(II) catalysts: microwave vs.conventional heating, Fatemeh Rajabi, Saghar Nourian, Sara Ghiassian, Alina M. Balu, Mohammad Reza Saidi, Juan Carlos Serrano-Ruiz and Rafael Luque, Green Chem., 2011, 13, 3282-3289
Recyclable copper catalysts based on imidazolium-tagged C2-symmetric bis(oxazoline) and their application in D–A reactions in ionic liquids, Zhi-Ming Zhou, Zhi-Huai Li, Xiao-Yan Hao, Xiao Dong, Xin Li, Li Dai, Ying-Qiang Liu, Jun Zhang, Hai-feng Huang, Xia Li and Jin-liang Wang, Green Chem., 2011, 13, 2963-2971
Amorphous carbon-silica composites bearing sulfonic acid as solid acid catalysts for the chemoselective protection of aldehydes as 1,1-diacetates and for N-, O– and S-acylations, Princy Gupta and Satya Paul, Green Chem., 2011, 13, 2365-2372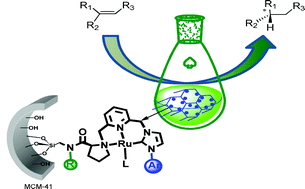
Recyclable mesoporous silica-supported chiral ruthenium-(NHC)NN-pincer catalysts for asymmetric reactions, Carolina del Pozo, Avelino Corma, Marta Iglesias and Félix Sánchez, Green Chem., 2011, 13, 2471-2481
Simple and recyclable ionic liquid based system for the selective decomposition of formic acid to hydrogen and carbon dioxide, M. E. M. Berger, D. Assenbaum, N. Taccardi, E. Spiecker and P. Wasserscheid, Green Chem., 2011, 13, 1411-1415
An efficient and heterogeneous recyclable palladium catalyst for chemoselective conjugate reduction of α,β-unsaturated carbonyls in aqueous medium, Dattatraya B. Bagal, Ziyauddin S. Qureshi, Kishor P. Dhake, Shoeb R. Khan and Bhalchandra M. Bhanage, Green Chem., 2011, 13, 1490-1494
Iron(III)-based ionic liquid-catalyzed regioselective benzylation of arenes and heteroarenes, Jian Gao, Jin-Quan Wang, Qing-Wen Song and Liang-Nian He, Green Chem., 2011, 13, 1182-1186
Pd immobilized on amine-functionalized magnetite nanoparticles: a novel and highly active catalyst for hydrogenation and Heck reactions, Fengwei Zhang, Jun Jin, Xing Zhong, Shuwen Li, Jianrui Niu, Rong Li and Jiantai Ma, Green Chem., 2011, 13, 1238-1243
Catalytic oxidative desulfurization with a hexatungstate/aqueous H2O2/ionic liquid emulsion system, Yuxiao Ding, Wenshuai Zhu, Huaming Li, Wei Jiang, Ming Zhang, Yuqing Duan and Yonghui Chang, Green Chem., 2011, 13, 1210-1216
Supported ionic liquid silica nanoparticles (SILnPs) as an efficient and recyclable heterogeneous catalyst for the dehydration of fructose to 5-hydroxymethylfurfural, Kalpesh B. Sidhpuria, Ana L. Daniel-da-Silva, Tito Trindade and João A. P. Coutinho, Green Chem., 2011, 13, 340-349
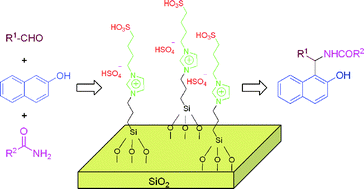 A silica gel supported dual acidic ionic liquid: an efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols, Qiang Zhang, Jun Luo and Yunyang Wei, Green Chem., 2010, 12, 2246-2254
A silica gel supported dual acidic ionic liquid: an efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols, Qiang Zhang, Jun Luo and Yunyang Wei, Green Chem., 2010, 12, 2246-2254
Highly recyclable, imidazolium derived ionic liquids of low antimicrobial and antifungal toxicity: A new strategy for acid catalysis, Lauren Myles, Rohitkumar Gore, Marcel Špulák, Nicholas Gathergood and Stephen J. Connon, Green Chem., 2010, 12, 1157-1162
 The latest issue of Green Chemistry is now available online.
The latest issue of Green Chemistry is now available online. The inside front cover highlights work by Richard Daniellou, Daniel Plusquellec and colleagues from the National School of Chemistry of Rennes and the European University of Brittany, France, who report aqueous solutions of facial amphiphilic carbohydrates as a sustainable media for organocatalyzed direct aldol reactions. Their system was applied to the direct aldol reaction of m-nitrobenzaldehyde with various cyclohexanones, and proceeded with high yields, shortened reaction times and improved diastereoselectivities.
The inside front cover highlights work by Richard Daniellou, Daniel Plusquellec and colleagues from the National School of Chemistry of Rennes and the European University of Brittany, France, who report aqueous solutions of facial amphiphilic carbohydrates as a sustainable media for organocatalyzed direct aldol reactions. Their system was applied to the direct aldol reaction of m-nitrobenzaldehyde with various cyclohexanones, and proceeded with high yields, shortened reaction times and improved diastereoselectivities. 










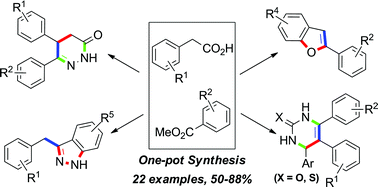
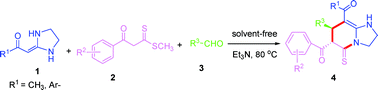

 A silica gel supported dual acidic ionic liquid: an efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols
A silica gel supported dual acidic ionic liquid: an efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols