Green Chemistry is proud to present the Green Chemistry Emerging Investigators Series, showcasing work being conducted by Emerging Investigators. This collection aims to highlight the excellent research being carried out by researchers in the early stages of their independent career from across the breadth of green chemistry. For more information about this series, click here
Green Chemistry is proud to present the Green Chemistry Emerging Investigators Series, showcasing work being conducted by Emerging Investigators. This collection aims to highlight the excellent research being carried out by researchers in the early stages of their independent career from across the breadth of green chemistry. For more information about this series, click here
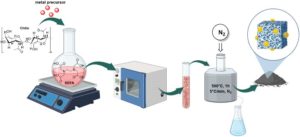
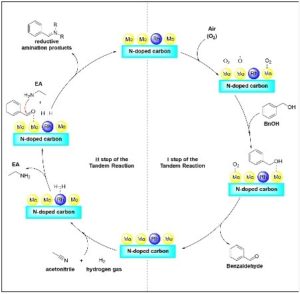
The most recent contribution to this series, a Paper entitled Orthogonal assisted tandem reactions for the upgrading of bio-based aromatic alcohols using chitin derived mono and bimetallic catalysts (Green Chem., 2024,26, 5221-5238, DOI: 10.1039/D3GC04848A), presents a tandem protocol for the valorisation of renewable alcohols derived from lignocellulosic biomass. The process involves an oxidation step followed by a reductive amination steps. By utilizing custom-made catalytic materials synthesized from renewable biopolymers derived from fishery waste, various aldehydes with potential applications as flavoring molecules were obtained, as well as secondary and tertiary amines that could serve as sustainable intermediates in the pharmaceutical industry. The authors explored the use of mechanochemistry for oxidizing solid alcohols.
Read our interview with the corresponding author below.
How would you set this article in a wider context?
While our primary focus lies in heterogeneous catalysis, this work carries significant implications for the broader context of sustainable chemistry and green technology. Specifically, it has the potential to impact industries involved in synthesizing flavouring molecules and pharmaceutical intermediates. Furthermore, our research aligns with ongoing efforts in biomass valorisation and waste management and reduction. From utilizing lignocellulosic waste via biomass-derived platform molecules as feedstocks to harnessing fishery waste as a renewable carbon and nitrogen source for catalytic material synthesis, our approach spans diverse avenues. Ultimately, this research contributes to global initiatives aimed at promoting sustainability and reducing the carbon footprint of the chemical industry.
What is the motivation behind this work?
Our main motivation is to offer potential solutions to address the requirements of the European Green Deal. Firstly, we aim to provide more eco-friendly alternatives by reducing or even eliminating the use of hazardous solvents through mechanochemistry. Secondly, we strive to develop safer chemical and technological solutions by utilizing renewable feedstocks and reducing dependency on fossil carbon. This includes employing renewable precursors derived from lignocellulosic waste or fishery waste for synthesizing chemicals and catalytic materials, respectively, thereby contributing to waste reduction.
 What aspects of this work are you most excited about at the moment and what do you find most challenging about it?
What aspects of this work are you most excited about at the moment and what do you find most challenging about it?
One aspect of this work that excites me the most is that we were able to conduct the oxidation reaction using air as the oxidizing agent, without pressurizing the autoclave reactors. This offers clear advantages for both safety and cost-efficiency of the protocol, potentially facilitating its scalability. Additionally, I’m thrilled about being able to perform the oxidation reaction under continuous-flow mechanochemical conditions in a twin-screw extruder, in this case using hydrogen peroxide as an oxidizing agent, but under solvent-free conditions. This provides another sustainable alternative for oxidizing solid benzyl-type alcohols. On the other hand, one of the most challenging aspects has been controlling the selectivity of the reductive amination step towards the desired products, an area we are continuously working on improving.
What is the next step? What work is planned?
This work has indeed sparked numerous new avenues for our ongoing research, particularly concerning the reductive amination of carbonyl-containing products and the potential applications in mechanochemistry. The use of green reducing agents for reduction and reductive amination reactions in mechanochemistry remains largely unexplored in the literature, posing a significant challenge. Nevertheless, we are highly motivated by some promising preliminary results in this area, although there is still much work to be done. It’s an exciting journey ahead!
Please describe your journey to becoming an independent researcher
My journey, as a Latin-American woman, to becoming an independent researcher has been filled with challenges, but it has been incredibly rewarding. From earning my bachelor’s degree in chemistry in Havana to completing my Ph.D. in Spain, and undertaking research stays in various universities across Europe, each experience has shaped me as a scientist and as a person. While relocating from my home country to Spain to pursue my Master’s and PhD degrees was one of the most challenging decisions I’ve made, it proved pivotal in shaping my academic trajectory.
Currently, I hold a post-doctoral position as a Marie-Curie Fellow at Università Ca’ Foscari di Venezia, Italy, under the Marie Sklodowska-Curie Cofund Grant Agreement No. 945361. Throughout my career, I have undertaken research stays at various institutions, including Università degli Studi Mediterranea di Reggio Calabria and Università degli Studi di Messina in Italy, as well as PSL Research University, Chimie ParisTech CNRS, in France. Additionally, I have gained valuable experience through research stays at Deasyl SA in Switzerland and KelAda Pharmachem Ltd. in Dublin, Ireland.
My research interests have been deeply rooted in the realm of materials science for different applications, with a strong emphasis on sustainability. My core objectives are to spearhead a transformative shift in the synthesis of materials. To tackle these goals, my approach centres on mechanochemistry and sustainable precursors to develop green and scalable protocols for tailoring nanomaterials with improved performance. In this line, I am dedicated to the use of wastes as a strategy to design new materials while enabling waste management and aligning with the circular economy.
Apart from the scientific challenges I eagerly embrace on a daily basis, one of the most daunting aspects I have faced has been navigating bureaucracy, especially coming from a Latin American country like Cuba. Yet, amidst these obstacles, I’ve been fortunate, especially to have crossed paths with remarkable individuals, mentors, and colleagues throughout my journey in every place I’ve been.
Can you share one piece of career-related advice or wisdom with other early career scientists?
If I could offer one piece of advice to fellow early-career scientists, it would be to embrace interdisciplinary collaborations. The most groundbreaking solutions often emerge from crossing disciplinary boundaries and exploring new perspectives. I am truly fortunate to have collaborated with outstanding scientists who have enriched my scientific knowledge and experience. Their expertise and insights have significantly contributed to my growth and development as a researcher.
Moreover, at this stage of my career, I’m increasingly engaged with students, something I find deeply fulfilling. For example, in the case of this contribution, collaborating with Francesco Zorzetto, who was once my student and is now my colleague, was truly an amazing experience. I can confidently say that I learned a great deal from him while working on this project. One key lesson that I consistently share with my students is that encountering negative results is normal: it’s part of the life of a researcher. What matters is perseverance, seeking alternatives, and returning to the laboratory the next day with renewed enthusiasm. Because perseverance and passion for what we do always yield rewards in the end.
Why did you choose to publish in Green Chemistry?
Choosing to publish in Green Chemistry was a no-brainer for me. It’s a prestigious journal known for its commitment to environmentally friendly chemical processes, which aligns perfectly with my research focus on sustainability.
Meet the author
 Daily Rodríguez-Padrón is a Marie-Curie Post-Doctoral researcher at Università Ca’ Foscari di Venezia, Italy (Marie Sklodowska-Curie Cofund Grant Agreement No. 945361). She earned her Bachelor’s degree in Chemistry from the University of Havana, Cuba, in 2013, and completed her Ph.D. in the Department of Organic Chemistry at the University of Cordoba, Spain, in 2020. In April 2020, she joined KelAda Pharmachem Ltd (Dublin, Ireland) as a visiting postdoctoral researcher, contributing to the Horizon 2020 Marie Skłodowska-Curie Action (MSCA) RISE project titled GreenX4Drug. Dr Rodríguez-Padrón has undertaken research stays in esteemed universities, including the Universita degli Studi Mediterranea di Reggio Calabria and the Università degli Studi di Messina in Italy, as well as the PSL Research University, Chimie ParisTech CNRS, in France. She serves on the Editorial Board of Sustainable Chemistry and has been invited as a Guest Editor for various journals, including Current Opinion in Green and Sustainable Chemistry, Topics in Current Chemistry, and Nanomaterials. Dr Rodríguez-Padrón has been laureated with the Dan David Prize 2019 in the field of Combatting Climate Change from Tel-Aviv University in Israel and the Green Talent Award 2020 from the German Federal Ministry of Education and Research. Her research primarily focuses on mechanochemistry, biomass valorisation, heterogeneous catalysis, and sustainability.
Daily Rodríguez-Padrón is a Marie-Curie Post-Doctoral researcher at Università Ca’ Foscari di Venezia, Italy (Marie Sklodowska-Curie Cofund Grant Agreement No. 945361). She earned her Bachelor’s degree in Chemistry from the University of Havana, Cuba, in 2013, and completed her Ph.D. in the Department of Organic Chemistry at the University of Cordoba, Spain, in 2020. In April 2020, she joined KelAda Pharmachem Ltd (Dublin, Ireland) as a visiting postdoctoral researcher, contributing to the Horizon 2020 Marie Skłodowska-Curie Action (MSCA) RISE project titled GreenX4Drug. Dr Rodríguez-Padrón has undertaken research stays in esteemed universities, including the Universita degli Studi Mediterranea di Reggio Calabria and the Università degli Studi di Messina in Italy, as well as the PSL Research University, Chimie ParisTech CNRS, in France. She serves on the Editorial Board of Sustainable Chemistry and has been invited as a Guest Editor for various journals, including Current Opinion in Green and Sustainable Chemistry, Topics in Current Chemistry, and Nanomaterials. Dr Rodríguez-Padrón has been laureated with the Dan David Prize 2019 in the field of Combatting Climate Change from Tel-Aviv University in Israel and the Green Talent Award 2020 from the German Federal Ministry of Education and Research. Her research primarily focuses on mechanochemistry, biomass valorisation, heterogeneous catalysis, and sustainability.