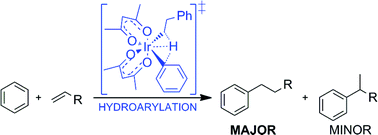
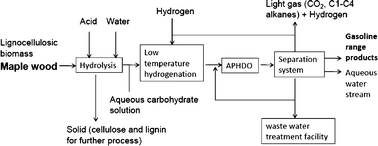
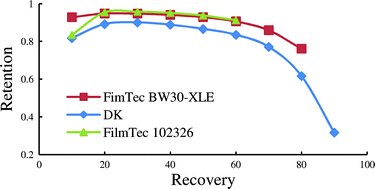
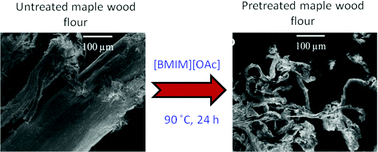
Philip Jessop and co-workers report tertiary amine solvents with switchable-hydrophilicity which have been applied to recycling polystyrene foam.
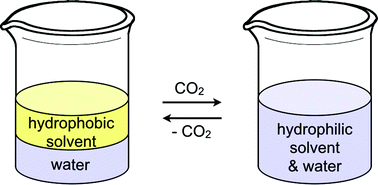
These switchable-hydrophilicity solvents (SHS) have very low miscibility with water and air, but are completely miscible in the presence of a CO2 atmosphere. Jessop and his team had previously reported using N,N,N’-tributylpentanamidine as a SHS which could easily be removed from organic products. However, N,N,N’-tributylpentanamidine is very difficult to synthesise and is not commercially available.
In this work, Jessop reports the use of several tertiary amines which are either commercially available or easily prepared. As well as investigating the factors which affect the rate of switching, the group also applied one of the amines studied to the recycling of polystyrene foam. The polystyrene foam was dissolved in one of the SHSs, and after addition of carbonated water formed dense polystyrene. In future, this could potentially make the transportation and recycling of polystyrene foam waste less energy intensive and more efficient.
Access this article for free by clicking on the link below:
Tertiary amine solvents having switchable hydrophilicity
Philip G. Jessop, Lisa Kozycz, Zahra Ghoshouni Rahami, Dylan Schoenmakers, Alaina R. Boyd, Dominik Wechsler and Amy M. Holland, Green Chem., 2011, DOI: 10.1039/C0GC00806K
For a related article, please see:
A solvent having switchable hydrophilicity
Philip G. Jessop, Lam Phan, Andrew Carrier, Shona Robinson, Christoph J. Dürr and Jitendra R. Harjani, Green Chem., 2010, 12, 809-814
DOI: 10.1039/B926885E