“This is the first demonstration of electrochemical hydrogenolysis,” says Richard G. Compton and co-workers.
The UK scientists at Oxford University showed a simple and rapid electrochemical hydrogenolysis process in room temperature ionic liquid requiring no hydrogen gas.
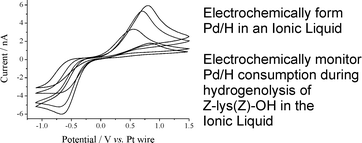
Hydrogenolysis of N,N¢-bis(benzyloxycarbonyl)-L-lysine was achieved by the electrochemical reduction of a labile proton at a Pd microelectrode,and the hydrogenolysis monitored by the size of the Pd/H oxidation peak. “The extension of this system to hydrogenation reactions should present no great problems, ” claims Compton and co-workers.
Read the article online:
Yao Meng, Leigh Aldous and Richard G. Compton, Green Chem., 2010, DOI: 10.1039/C0GC00450B