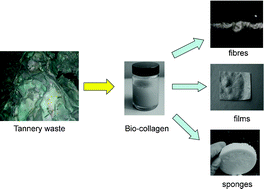
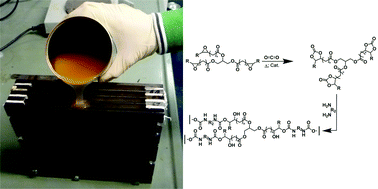
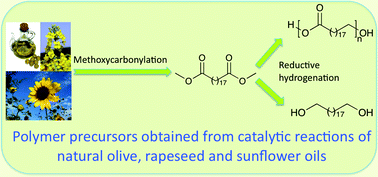
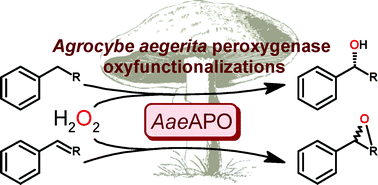
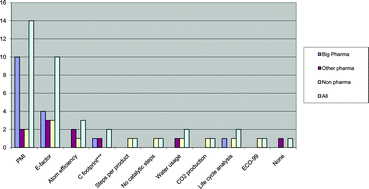
Scientists from Germany have optimized and assessed the ecological performance of a whole-cell two-liquid phase biocatalytic epoxidation of styrene.
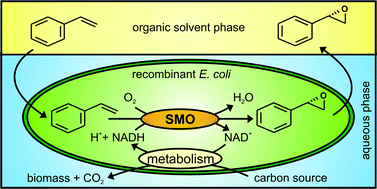
The authors also replaced the carbon/energy source glucose with glycerol as the latter is a waste product from the biodiesel and soap industries and thus cheap and abundant. However, the use of glycerol was found to reduce the overall ecological and economic performance of the process. The work presented here by Bühler and co-workers illustrates the capability of these assessments to identify critical process parameters and to enable systematic development towards industrial implementation.
This article is free to access until the 24th February 2012! Click on this link below to find out more…
Systematic optimization of a biocatalytic two-liquid phase oxyfunctionalization process guided by ecological and economic assessment, Daniel Kuhn, Mattijs K. Julsing, Elmar Heinzle and Bruno Bühler, Green Chem., 2012, DOI: 10.1039/C2GC15985F
You may find this article of interest too – also free to access until the 24th February 2012, so why not take a look…
Intensification and economic and ecological assessment of a biocatalytic oxyfunctionalization process, Daniel Kuhn, Muhammad Abdul Kholiq, Elmar Heinzle, Bruno Bühler and Andreas Schmid, Green Chem., 2010, 12, 815-827
Keep up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts!