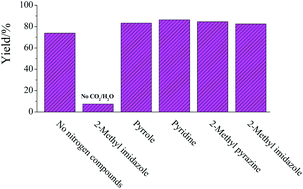
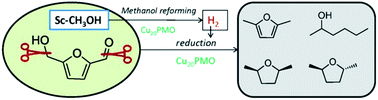
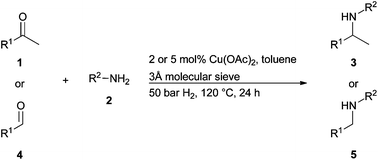
Scientists from Denmark and the USA have achieved efficient conversion of 5-hydroxymethylfurfural (HMF) to valuable chemicals over a Cu-doped porous metal oxide catalyst in supercritical methanol.
HMF (readily obtained from hexose sugars) has been identified as a key platform compound to generate useful renewable chemicals for the fuel industry, such as 2,5-dimethylfuran (DMF) and 2,5-dimethyltetrahydrofuran (DMTHF). However, achieving selective transformation of HMF to a specific product and preventing the formation of undesired side-products remains a challenge.
This article is free to access until the 28th September 2012! Click on this link below to fine out more…
One-pot reduction of 5-hydroxymethylfurfural viahydrogen transfer from supercritical methanol, Thomas S. Hansen, Katalin Barta, Paul T. Anastas, Peter C. Ford and Anders Riisager, Green Chem., 2012, 14, 2457-2461
You may also be interested in reading this article – free to access for 2 weeks:
Synergy of boric acid and added salts in the catalytic dehydration of hexoses to 5-hydroxymethylfurfural in water, Thomas S. Hansen, Jerrik Mielby and Anders Riisager, Green Chem., 2011, 13, 109-114
Stay up-to-date with the latest news and content in Green Chemistry by registering for our free table of contents alerts.












 Raymond Robertson
Raymond Robertson