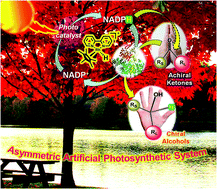
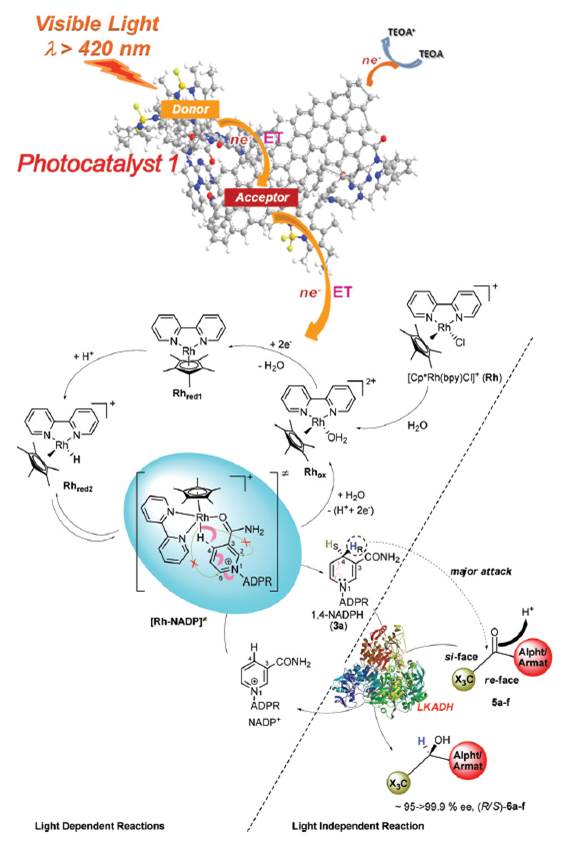
 The asymmetric, enzymatic reduction of ketones has been enhanced with a graphene derived light harvesting photocatalyst. Typically the use of reducing enzymes for specialty chemical synthesis is restricted by the cost of the redox cofactor. In this example the enzyme cofactor is recycled via a rhodium complex. The energy needed to do this is delivered by the chlorophyll mimicking graphene. Enantioselectivity to the resulting alcohols is high, and applicable to both aliphatic and aromatic ketones.
The asymmetric, enzymatic reduction of ketones has been enhanced with a graphene derived light harvesting photocatalyst. Typically the use of reducing enzymes for specialty chemical synthesis is restricted by the cost of the redox cofactor. In this example the enzyme cofactor is recycled via a rhodium complex. The energy needed to do this is delivered by the chlorophyll mimicking graphene. Enantioselectivity to the resulting alcohols is high, and applicable to both aliphatic and aromatic ketones.
The scientists from KRICT responsible for this research believe that artificial photosynthesis using functionalised graphene shows promise for energy generation and sustainable chemical production in the near future, with applications including carbon dioxide sequestering reactions already proven as viable.
Check out the full article – online now!