The 10 most-accessed Green Chemistry articles between July and September 2013 were as follows:
Synthesis of thioesters through copper-catalyzed coupling of aldehydes with thiols in water
Chih-Lun Yi, Yu-Ting Huang and Chin-Fa Lee
Green Chem., 2013,15, 2476-2484, DOI: 10.1039/C3GC40946E, Paper
Highly efficient iron(0) nanoparticle-catalyzed hydrogenation in water in flow
Reuben Hudson, Go Hamasaka, Takao Osako, Yoichi M. A. Yamada, Chao-Jun Li, Yasuhiro Uozumi and Audrey Moores
Green Chem., 2013,15, 2141-2148, DOI: 10.1039/C3GC40789F, Paper
Iodine-mediated arylation of benzoxazoles with aldehydes
Yew Chin Teo, Siti Nurhanna Riduan and Yugen Zhang
Green Chem., 2013,15, 2365-2368, DOI: 10.1039/C3GC41027G, Communication
Polymer anchored Cu(II) complex: an efficient and recyclable catalytic system for the one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles starting from anilines in water
Susmita Roy, Tanmay Chatterjee and Sk. Manirul Islam
Green Chem., 2013,15, 2532-2539, DOI: 10.1039/C3GC41114A, Paper
Multicomponent reactions in unconventional solvents: state of the art
Yanlong Gu
Green Chem., 2012,14, 2091-2128, DOI: 10.1039/C2GC35635J, Critical Review
Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation
Sarina Sarina, Eric R. Waclawik and Huaiyong Zhu
Green Chem., 2013,15, 1814-1833, DOI: 10.1039/C3GC40450A, Tutorial Review
Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation
Jonathan G. Huddleston, Ann E. Visser, W. Matthew Reichert, Heather D. Willauer, Grant A. Broker and Robin D. Rogers
Green Chem., 2001,3, 156-164, DOI: 10.1039/B103275P, Paper
Deconstruction of lignocellulosic biomass with ionic liquids
Agnieszka Brandt, John Gräsvik, Jason P. Hallett and Tom Welton
Green Chem., 2013,15, 550-583, DOI: 10.1039/C2GC36364J, Critical Review
Hydrolysis of cellulose to glucose by solid acid catalysts
Yao-Bing Huang and Yao Fu
Green Chem., 2013,15, 1095-1111, DOI: 10.1039/C3GC40136G, Tutorial Review
Catalytic conversion of biomass to biofuels
David Martin Alonso, Jesse Q. Bond and James A. Dumesic
Green Chem., 2010,12, 1493-1513, DOI: 10.1039/C004654J, Critical Review
Take a look at the articles and then let us know your thoughts and comments below.
Fancy submitting your own work to Green Chemistry? You can submit online today, or email us with your ideas and suggestions.











 The front cover this month (pictured left) features work by Peter C. K. Lau and co-workers from Quebec, Canada. In their work they engineer sinapic acid decarboxylaseas an alternative to chemistry-based or thermal decarboxylation to produce canolol from canola meal.
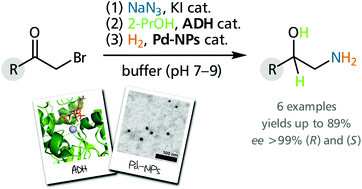
The front cover this month (pictured left) features work by Peter C. K. Lau and co-workers from Quebec, Canada. In their work they engineer sinapic acid decarboxylaseas an alternative to chemistry-based or thermal decarboxylation to produce canolol from canola meal. The inside front cover this month (pictured right) features work by Joerg Schrittwieser, Frank Hollmann and co-workers from Deltf, The Netherlands. In their work they show how the one-pot combination of alcohol dehydrogenase (ADH) and palladium nanoparticle (Pd-NP) catalysis provides access to aromatic 1,2-amino alcohols in high yields and excellent optical purities.
The inside front cover this month (pictured right) features work by Joerg Schrittwieser, Frank Hollmann and co-workers from Deltf, The Netherlands. In their work they show how the one-pot combination of alcohol dehydrogenase (ADH) and palladium nanoparticle (Pd-NP) catalysis provides access to aromatic 1,2-amino alcohols in high yields and excellent optical purities.


 The front cover this month (pictured left) features work by Wouter De Soute and co-workers from Ghent, Belgium. In their work, they show that shifting from batch to continuous pharmaceutical tablet manufacturing results in a significant reduction in natural resource extraction.
The front cover this month (pictured left) features work by Wouter De Soute and co-workers from Ghent, Belgium. In their work, they show that shifting from batch to continuous pharmaceutical tablet manufacturing results in a significant reduction in natural resource extraction.  The inside front cover this month (pictured right) features work by Davit Zargarian and co-workers from Quebec, Canada. In their work they reveal a new one-pot method for the efficient and atom-economical synthesis of POCOP-type pincer complexes of divalent nickel that serve as pre-catalysts for various catalytic transformations.
The inside front cover this month (pictured right) features work by Davit Zargarian and co-workers from Quebec, Canada. In their work they reveal a new one-pot method for the efficient and atom-economical synthesis of POCOP-type pincer complexes of divalent nickel that serve as pre-catalysts for various catalytic transformations.




 The inside front cover this month (pictured right) features work by Jorge Beltramini and co-workers from Brisbane, Australia. In their work they utilize high resolution NMR to explain the depolymerisation mechanism of cellulose during acid treatment and milling.
The inside front cover this month (pictured right) features work by Jorge Beltramini and co-workers from Brisbane, Australia. In their work they utilize high resolution NMR to explain the depolymerisation mechanism of cellulose during acid treatment and milling.