Issue 1 of Green Chemistry is now available to read online.
This year we celebrate our milestone of 15 years of publication, and in issue 1 you can read an Editorial from all of Green Chemistry’s Chairs of the Editorial board and Scientific Editors marking the occasion.
We have also put together a special web collection, with contributions from authors who have had highly cited articles from each of the past 15 years : 15 Years of Green Chemistry
 The front cover this month (pictured left) features work by Brian Davison and co-workers. In their work they investigate the mechanism of biomass breakdown. Understanding this process should lead to more efficient use of biomass.
The front cover this month (pictured left) features work by Brian Davison and co-workers. In their work they investigate the mechanism of biomass breakdown. Understanding this process should lead to more efficient use of biomass.
Read the full article:
Common processes drive the thermochemical pretreatment of lignocellulosic biomass
Paul Langan, Loukas Petridis, Hugh M. O’Neill, Sai Venkatesh Pingali, Marcus Foston, Yoshiharu Nishiyama, Roland Schulz, Benjamin Lindner, B. Leif Hanson, Shane Harton, William T. Heller, Volker Urban, Barbara R. Evans, S. Gnanakaran, Arthur J. Ragauskas, Jeremy C. Smith and Brian H. Davison
Green Chem., 2014, 16, 63-68, DOI: 10.1039/C3GC41962B
 The inside front cover this month (pictured right) features work by Kevin Moeller and co-workers from Missouri, USA. In their work they set up a simple solar-electrochemical reaction to recycle Os(VIII)-, TEMPO-, Ce(IV)-, Pd(II)-, Ru(VIII)-, and Mn(V)-oxidants.
The inside front cover this month (pictured right) features work by Kevin Moeller and co-workers from Missouri, USA. In their work they set up a simple solar-electrochemical reaction to recycle Os(VIII)-, TEMPO-, Ce(IV)-, Pd(II)-, Ru(VIII)-, and Mn(V)-oxidants.
Read the full article:
Sunlight, electrochemistry, and sustainable oxidation reactions
Bichlien H. Nguyen, Alison Redden and Kevin D. Moeller
Green Chem., 2014, 16, 69-72, DOI: 10.1039/C3GC41650J
Both of these articles are free to access for 6 weeks!
Keep up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts.













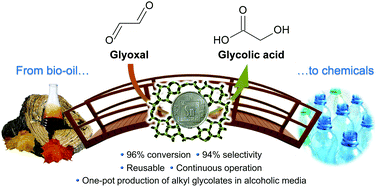

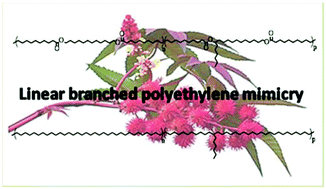

 The front cover this month (pictured left) features a review by Jesse Hensley and co-workers from Golden, Colorado. In their article they focus on recent model compound studies of catalysts for hydrodeoxygenation of biomass pyrolysis products, with an emphasis on mechanisms, reaction networks, and structure–function relationships.
The front cover this month (pictured left) features a review by Jesse Hensley and co-workers from Golden, Colorado. In their article they focus on recent model compound studies of catalysts for hydrodeoxygenation of biomass pyrolysis products, with an emphasis on mechanisms, reaction networks, and structure–function relationships. The inside front cover this month (pictured right) features work by Andreas Heyden and co-workers from Columbia, South Carolina. In their work they report a theoretical study of the effects of various solvents on the mechanism of the hydrodeoxygenation of propanoic acid over Pd(111).
The inside front cover this month (pictured right) features work by Andreas Heyden and co-workers from Columbia, South Carolina. In their work they report a theoretical study of the effects of various solvents on the mechanism of the hydrodeoxygenation of propanoic acid over Pd(111).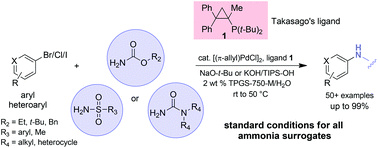
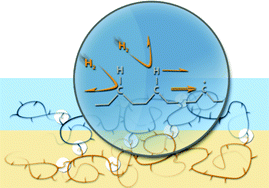
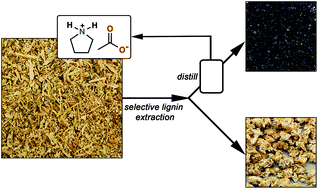

 The front cover this month (pictured left) features work by Brian Davison and co-workers. In their work they investigate the mechanism of biomass breakdown. Understanding this process should lead to more efficient use of biomass.
The front cover this month (pictured left) features work by Brian Davison and co-workers. In their work they investigate the mechanism of biomass breakdown. Understanding this process should lead to more efficient use of biomass. The inside front cover this month (pictured right) features work by Kevin Moeller and co-workers from Missouri, USA. In their work they set up a simple solar-electrochemical reaction to recycle Os(VIII)-, TEMPO-, Ce(IV)-, Pd(II)-, Ru(VIII)-, and Mn(V)-oxidants.
The inside front cover this month (pictured right) features work by Kevin Moeller and co-workers from Missouri, USA. In their work they set up a simple solar-electrochemical reaction to recycle Os(VIII)-, TEMPO-, Ce(IV)-, Pd(II)-, Ru(VIII)-, and Mn(V)-oxidants.