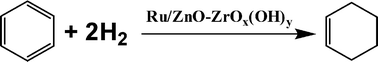Iron usually rusts in the presence of water, however, the protective polymers developed by Yasuhiro Uozumi and Audrey Moores prevent the rusting process, allowing iron to act as an efficient and selective catalyst for hydrogenation with water as the solvent © Shutterstock
An international team of chemists has reported a clean and green way to perform one of the most important industrial reactions for pharmaceutical and petrochemical synthesis.
Platinum group metals are currently the catalysts of choice for hydrogenations due to their high activity. However, they are also expensive, toxic and very rare. Now, in a joint project between McGill University, Canada, and the RIKEN Institute, Japan, a polymer supported iron catalyst has demonstrated excellent performance as a hydrogenation catalyst in the most environmentally-friendly of reaction mediums – water.
Read what Audrey Moores and Jianliang Xiao had to say about the research in the Chemistry World story!
Read the original research published in Green Chemistry:
Highly efficient iron(0) nanoparticle-catalyzed hydrogenation in water in flow, Reuben Hudson, Go Hamasaka, Takao Osako, Yoichi M. A. Yamada, Chao-Jun Li, Yasuhiro Uozumi and Audrey Moores, Green Chem., 2013, DOI: 10.1039/C3GC40789F













 The ACS Green Chemistry Institute®’s
The ACS Green Chemistry Institute®’s 

 Although some of the waste ash produced from the combustion of biomass is currently used in construction, most of it ends up in landfill. Therefore, extracting alkali silicates, which can be used in cement, detergents, catalysts and catalyst supports, is one way of reusing the potentially huge quantities of ash due to be produced in the future.
Although some of the waste ash produced from the combustion of biomass is currently used in construction, most of it ends up in landfill. Therefore, extracting alkali silicates, which can be used in cement, detergents, catalysts and catalyst supports, is one way of reusing the potentially huge quantities of ash due to be produced in the future.