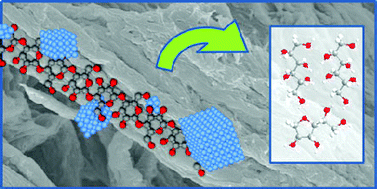
A magnetically separable nanomaterial has been developed which showed good photocatalytic activity under visible light in the selective transformation of malic acid in aqueous solution.
Titanium dioxide (TiO2) is well known as a photocatalyst, but its application in various processes is hindered due to several limitations. The most important of these limitations is its lack of photocatalytic activity under visible light. In addition, efficient separation of the catalyst once the reaction is complete can be difficult, with additional filtration steps and/or a tedious work-up required.
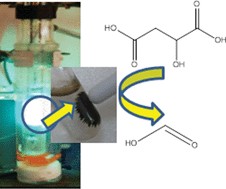
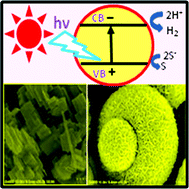
However, in this work scientists from Spain and the USA led by Rafael Luque and Rajender Varma have developed a novel nanomaterial which overcomes these problems. The TiO2-guanidine-(Ni,Co)Fe2O4nanomaterial was shown to have good activity in the selective transformation of malic acid. Not only did the reaction proceed well under visible light, the material was also shown to have superior activity over the commercial catalyst Degussa P25. The catalyst could be separated from the reaction mixture simply by using a magnet and the catalyst could be reused several times, preserving most of its activity.
To read more, please click on the link below. This article is free to access until the 30th September 2011!
Magnetically separable nanocomposites with photocatalytic activity under visible light for the selective transformation of biomass-derived platform molecules, Alina M. Balu, Babita Baruwati, Elena Serrano, Jaume Cot, Javier Garcia-Martinez, Rajender S. Varma and Rafael Luque, Green Chem., 2011, DOI: 10.1039/C1GC15692F
Click here to read Anna Simpson’s recent interview with Rafael Luque!