Scientists from New Zealand have used food additive-derived ionic liquids for extracting wood lignin.
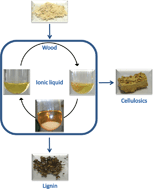
Wood cellulose, the most abundant biopolymer on earth, has great potential as a renewable feedstock, but in order to ‘unlock’ this potential, the various components of wood cellulose need to separated and processes individually. The three main components are cellulose, hemicellulose and lignin. However, current methods used to separate these components, for example Kraft pulping, have many draw backs such as high operating temperatures and pressures and many sequential steps.
In this work, André Pinkert and his team used food additive-derived ionic liquids for seperating wood lignin and looked at the influence of selected parameters on the process. In one gentle step, an extraction efficiency of e= 0.43 of wood lignin was achieved which increased to e= 0.60 in the presence of a co-solvent. The gentle conditions employed here did not decrease the crystallinity of the wood sample, and resulted in lignin with both a larger molar mass and a more uniform molar mass distribution compared to commercially available Kraft lignin.
Click the links below to read more. This article is free to access until the 18th November 2011!
Extracting wood lignin without dissolving or degrading cellulose: investigations on the use of food additive-derived ionic liquids, André Pinkert, Dagmar F. Goeke, Kenneth N. Marsh and Shusheng Pang, Green Chem., 2011, DOI: 10.1039/C1GC15671C