Over the past 25 years, Green Chemistry has provided a unique forum for the publication of innovative research on the development of alternative sustainable technologies, efficient utilisation of resources and the concomitant minimisation of waste. We are delighted to bring together a very special issue containing articles by members of the green chemistry community as well as past and present Green Chemistry Board members, to mark and celebrate our first 25 years.
Among the contributions to this themed collection is a Paper on the chemical valorisation of biomass derived furanics and carboxylic acids over niobium-based catalysts (DOI: 10.1039/D4GC00207E).
Read our interview with Margarida M. Antunes, one of the corresponding authors.
Could you briefly explain the focus of your article to the non-specialist (in two or three sentences only)?
How would you set this article in a wider context?
Niobium based oxides are known to have a lot of advantages, such as water-tolerance. This is very important since water is a co-product of the biomass conversion processes we intend to explore. Moreover, they possess Brønsted and Lewis acidity required for these conversion processes and have shown superior catalytic performance in relation to other metal oxides.
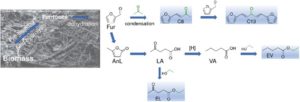
In collaboration with Dr Nicola Pinna and Dr Patrícia Russo (Humboldt University Berlin), niobium oxide nanoparticles embedded in a mesoporous silica matrix were synthesised by a relatively simple methodology. Our group investigated the catalytic properties. The prepared materials were stable in recycling runs in various reactions, such as for the aldol condensation of furfural with acetone, and for esterification reactions of alpha-angelica lactone, levulinic and valeric acid, which are all furfural derivatives. Moreover, they showed high acetone and ethanol consumption efficiency, which is important for an integrated biorefinery concept and sustainability.
What is the motivation behind this work?
Our group enjoys the fascinating field of Catalysis, which is crucial to the Chemical Industry. We explore selective and stable catalysts to efficiently promote target chemical reactions under moderate conditions, which may lead to more sustainable processes.
What aspects of this work are you most excited about at the moment and what do you find most challenging about it?
It was exciting to realize that indeed the niobium oxide nanoparticles embedded in a mesoporous silica (especially SiNb42 and SiNb75) could significantly increase the selectivity towards our main product, 4-(furan-2-yl)but-3-en-2-one (C8), in relation to the niobia nanomaterial Nb2O5, or in relation to a commercial micro Nb2O5 (which led to sluggish results) or yet in relation to a Nb2O5/TUD-1 composite using an equivalent atomic amount of Nb, prepared by embedding the Nb2O5 nanoparticles in a mesoporous TUD-1 type silica. Moreover, the Nb2O5 alone were partially deactivated in recycling runs, albeit when embedded in a mesoporous silica (using silica tetrachloride), their stability was enhanced. However, for Nb2O5/TUD-1 there was observed deactivation. The reasons for such behaviour are still not clear. The inherent properties of each material play an important role in their catalytic performance.
What is the next step? What work is planned?
Our next step is trying to use waste biomass sources instead of model substrates by using the acquired knowledge. This is of course a challenge, but we hope to count on the collaboration of scientists from the academia and industry, attempting to come closer to practical application.
Please describe your journey to becoming part of the Green Chemistry community
Since I integrated the associated laboratory CICECO, at the University of Aveiro, in 2008, I have been engaged in developing research work in the field of catalysis, with a mindset on sustainability and Green Chemistry. Developing research with this mindset is a reason for feeling proud.
Why did you choose to publish in Green Chemistry?
Green Chemistry is a prestigious journal, internationally recognised and read by many scientists, and valorises research that aims to reduce the environmental impact associated with the chemistry industry. In this sense, we thought that Green Chemistry was the best choice for our manuscript.
What do you think the Green Chemistry journal has done well in the past 25 years, and what do you think are the main challenges our community will face in the next 25 years?
During the last 25 years, the Green Chemistry journal has evolved according to the demands for sustainability. Several reviews and perspective papers have been discussed concerning the use of greener alternatives for fossil fuels. Environmental issues and sustainable solutions have been debated through life cycle assessment studies and techno economic studies. Some studies are promising and reliable experimentally, but the costs of the processes are still high. Sustainable processes for producing biofuels and biobased chemicals continues to demand much investigation. In the coming years, the way of use of fossil fuels and the supply chains should be rethought, which calls for the Green Chemistry community.
Meet the corresponding author
 |
Margarida M. Antunes received a Licentiate degree in Industrial Chemistry from the University of Coimbra, Portugal, in 2004, where she studied the polymorphism of erythritol and threitol using differential scanning calorimetry and thermomicroscopy. In 2008, she earned a Master´s degree in Chemistry of Natural Products from the University of Aveiro (UA), where she synthesised and characterised pharmaceutical compounds. Margarida proceeded to doctoral studies at the UA, having concluded her PhD thesis in 2012, on the conversion of polysaccharides to furanic derivatives using either ionic liquids or heterogeneous acid catalysts. In the same year, she was awarded a post-doc grant from the FCT (Portuguese Foundation for Science and Technology) and in 2019 she became researcher at the UA, CICECO – Aveiro Institute of Materials. She investigates the catalytic conversion of several types of biomass derivatives to valuable bio-based products via heterogeneous catalytic routes. For example, D-fructose for food and non-food applications, fuel blends synthesised via condensation between biomass derived compounds (e.g. furfural) and glycerol, and gamma-valerolactone which is a promising fuel additive and intermediate for transportation fuels. She develops multifunctional heterogeneous catalysts to carry out multiple reactions (e.g. acid and reduction) in one-pot synthesis strategies. The heterogeneous catalysts include bimetallic, micro- and mesoporous materials prepared via different procedures, such as hydrothermal syntheses and top-down modifications (leading to hierarchical materials). Besides developing catalysts, Margarida is concerned about the energy efficiency of processes and has investigated different energy supply methods. One of her strong motivations is to study the sustainable repurposing of undesirable waste biomass sources to drop-in-fuels with reduced CO2 emissions compared to fossil fuels, via different catalytic strategies. |