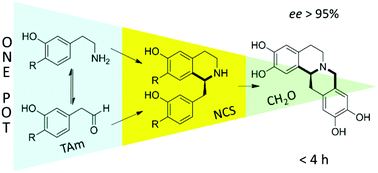
Researchers at UCL, UK, have developed a novel use of one-pot, multi-step enzymatic catalysis for the synthesis of chiral alkaloids. Transaminase (TAm) and norcoclaurine synthase (NCS) were employed as catalysts. In the synthesis of the tetrahydroprotoberberine product two carbon-carbon bonds are created under mild conditions with good enantiomeric purity (ee >95%), all in a short duration (3.5 hours).
The successive Pictet-Spengler cyclisation reactions provide an atom economic approach and high enantioselectivity to the formation of this valuable class of products. The authors of this work recognise the “remarkable potential of in vitro biocatalysis for the formation of complex chiral compounds“, which is increasingly important to contemporary green chemistry.
This article is open access and available to everyone to read for free:
One-pot triangular chemoenzymatic cascades for the synthesis of chiral alkaloids from dopamine
B. R. Lichman, E. D. Lamming, T. Pesnot, J. M. Smith, H. C. Hailes and J. M. Ward
Green Chem., 2015, Advance Article. DOI: 10.1039/C4GC02325K










