Chinese scientists have developed a new approach to synthesize acridones viacopper-catalyzed C–C bond cleavage and intramolecular cyclization.
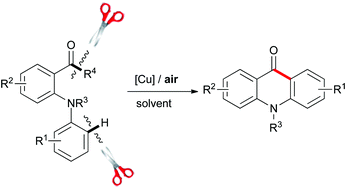
In this work, Wang Zhou and colleagues from Xiangtan University, China, used a copper catalyst and air as the oxidant to synthesize acridones through a C–C bond cleavage and intramolecular cyclization, under neutral conditions. This reaction provides an alternative strategy for C–C cleavage.
Read the full article for free until the 11th December 2012:
Copper-catalyzed C–C bond cleavage and intramolecular cyclization: an approach toward acridones, Wang Zhou, Youqing Yang, Yong Liu and Guo-Jun Deng, Green Chem., 2012, DOI: 10.1039/C2GC36502B
You may also be interested in these articles – free to access for 2 weeks:
Copper(II) catalysis provides cyclohexanone-derived propargylamines free of solvent or excess starting materials: sole by-product is water, Conor J. Pierce and Catharine H. Larsen, Green Chem., 2012, 14, 2672-2676
An efficient copper-catalyzed formation of highly substituted pyrazoles using molecular oxygen as the oxidant, Mamta Suri, Thierry Jousseaume, Julia J. Neumann and Frank Glorius, Green Chem., 2012, 14, 2193-2196
A highly efficient Cu-catalyst system for N-arylation of azoles in water, Deping Wang, Fuxing Zhang, Daizhi Kuang, Jiangxi Yu and Junhua Li, Green Chem., 2012, 14, 1268-1271
Microwave-assisted solvent- and ligand-free copper-catalysed cross-coupling between halopyridines and nitrogen nucleophiles, Zhen-Jiang Liu, Jean-Pierre Vors, Ernst R. F. Gesing and Carsten Bolm, Green Chem., 2011, 13, 42-45
Stay up-to-date with the latest news and content in Green Chemistry by registering for our free table of contents alerts.










