Chinese scientists report the selective oxidation of organic sulfides with oxone to give either sulfoxides or sulfones.
Sulfoxides and sulfones are important synthetic reagents widely used in the preparation of biologically active compounds. However, their selective synthesis from sulfides is still a challenge, and although methods for achieving this are available they tend to still a require catalyst or promoter.
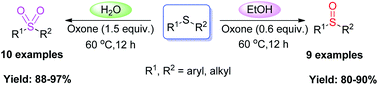
This article is free to access until the 30th March 2012! Click on the link below to find out more…
Catalyst-free approach for solvent-dependent selective oxidation of organic sulfides with oxone, Bing Yu, An-Hua Liu, Liang-Nian He, Bin Li, Zhen-Feng Diao and Yu-Nong Li, Green Chem., 2012, DOI: 10.1039/C2GC00027J
Stay up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts.










