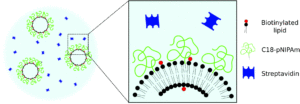
Lipid bilayers are membranes consisting of two layers of lipid molecules. They have attracted an enormous interest from the research community, as they form the external envelope of cells. In three dimensions, these lipid bilayers can form vesicles, and such systems are currently used as models for many studies of cell signalling. In addition, they can be loaded with chemicals and used as drug delivery carriers. The performance of a drug delivery system formed by vesicles relies on their stability or, in other words, how much they tend to aggregate and in what manner. A system of vesicles where all of them aggregate into a very large cluster before they are even injected in the body will be of no use as it will not reach its target. On the other hand, if the vesicles all remain apart from each other indefinitely the dose applied in the target area might not be large enough to be effective.
In this publication, the authors design a vesicle system that enables full control over the aggregation of vesicles. A combination of reversible and irreversible linkers is employed to form the vesicle aggregates, and such combination allows control of their size by setting the appropriate temperature program. The authors envisage that their method will be a useful tool for investigating membrane fusion phenomena or inter-membrane interactions, which are of high relevance in biological processes.
Comments from the authors:
- We found that optimal conditions for self-limiting aggregation (defined in the text) are found when the lateral linker diffusion time is much shorter compared to the vesicle collision time. Experimentation at low vesicle concentration is therefore crucial/key.
- Continued aggregation takes place at high temperatures (>32 degrees C) at high fractions of biotinylated lipids in combination with a high concentration of streptavidin. This aggregation can be stopped by lowering the temperature (C18-pNIPAm swells and prevents further aggregation). Rather than self-limiting aggregation, we have then obtained an aggregation start and stop mechanism using the C18-pNIPAm linker.
- Optimizing the protocol for C18-pNIPAm synthesis, and varying the length of the pNIPAm polymer could further improve the control on final aggregation sizes.
- Additionally, further control on the aggregation could be obtained varying the temperature steps. For temperature steps lower than 40 degrees C (but >32 degrees C), aggregation of vesicles is slower.
- This work not only showcases the effect of combining biotin/streptavidin and C18-pNIPAm, but can also serves as a proof of principle for vesicle aggregation with other combinations of linkers, of which one of the linkers is reversible.
Citation to the paper: Step-wise linking of vesicles by combining reversible and irreversible linkers – towards total control on vesicle aggregate sizes, N. de Lange, F. A. M. Leermakers, and J. M. Kleijn. Soft Matter, 2020,16, 6773. DOI: 10.1039/D0SM00995D.
To read the full article click here!
About the web writer
Dr Nacho Martin-Fabiani (@FabianiNacho) is a UKRI Future Leaders Fellow and Senior Lecturer in Materials Science at Loughborough University, UK.