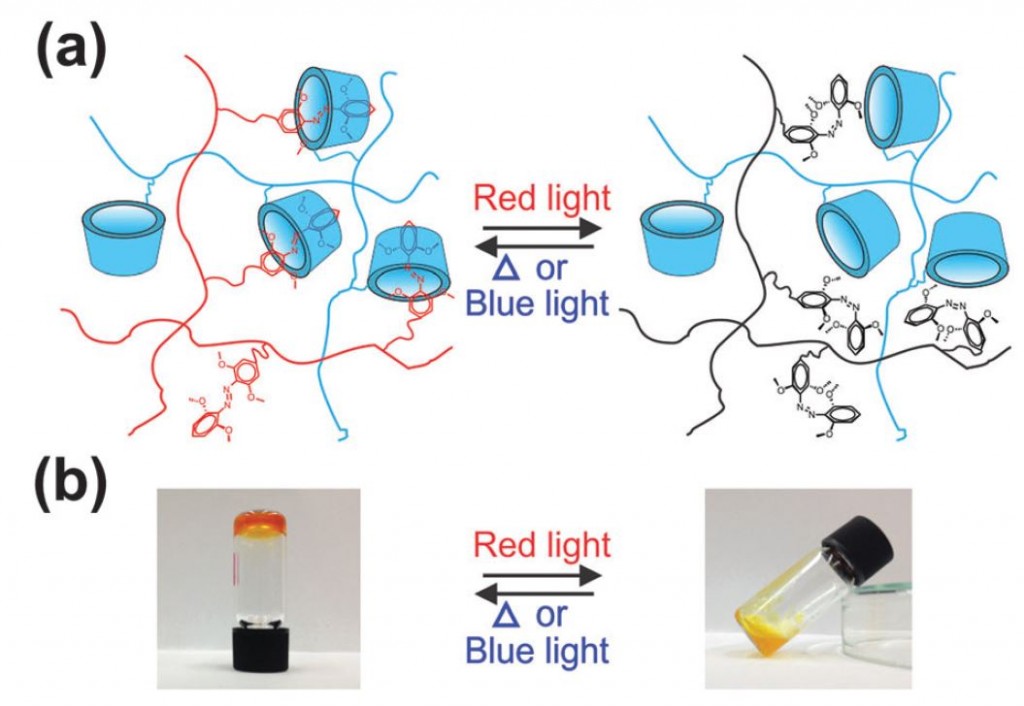
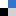Hydrogel synthesis is a well-established method of incorporating cells, proteins, or drugs into a biocompatible polymer network. The majority of hydrogels are stimulated and cross-linked by a chemical reaction or UV light, but this can limit their use in vivo as these methods damage healthy cells. Using red light-stimulated hydrogels would be better suited for the therapeutic use of hydrogels, as it creates little photo damage to cells and red light can penetrate deeper into tissue than other light wavelengths. Such a hydrogel was developed by a research group at the Max Planck Institute for Polymer Research in Germany and is described in detail in a recently published full article in Soft Matter. The group describes a synthetic method of hydrogel formation using methoxy-modified azobenzene that activates the polymer complex under red light.
The hydrogel synthesis is based on the spontaneous formation of a supramolecular complex by combining an azobenzene (mAzo) derivative and β-cyclodextrin (β-CD). The two chemical species were individually grafted onto a poly(acrylic acid) (PAA) polymer backbone and mixed to form the red light-activated hydrogel. During exposure to red light, the azobenzene moiety in mAzo underwent isomerization from the trans to cis state, which was not hindered by the presence of β-CD. The process is reversible, with heat or blue light returning mAzo to the trans state. While the mAzo polymer remained in the trans state, the mAzo/β-CD complex maintained a gelatin formation, but once altered to the cis state by red light, the hydrogel destabilized and became a liquid. This sol-to-gel transition is explained by the high binding constant between trans mAzo and β-CD (Ka = 1546 M-1) compared to low binding constant between cis mAzo and β-CD (Ka = 82.1 M-1).
To demonstrate the hydrogel’s utility for medical applications, the hydrogel was loaded with the protein, bovine serum albumin (BSA). Exposure to red light dissolved the hydrogel and released ~83% of the protein into solution. If a piece of porcine tissue was placed between the red light source and the protein laden hydrogel, mAzo was still capable of transitioning to the cis state for disassembly of the gel and release of the BSA. The ability of the hydrogel to be activated through tissue using non-invasive and non-detrimental red light is an impressive step for the development of therapeutic and light-stimulated polymers for controlled drug or protein release.
See the full Soft Matter article here:
Supramolecular hydrogels constructed by red-light-responsive host–guest interactions for photo-controlled protein release in deep tissue
Dongsheng Wang, Manfred Wagner, Hans-Jürgen Butt, and Si Wu
Soft Matter, 2015, Advance Article
DOI: 10.1039/C5SM01888A
 Dr. Morgan M. Stanton is currently a postdoctoral researcher at the Max Planck Institute for Intelligent Systems in Stuttgart, Germany. She completed her Ph.D. in Chemistry from Worcester Polytechnic Institute in 2014. Read more about Morgan’s research publications here or you can follow her on Twitter @morg368.
Dr. Morgan M. Stanton is currently a postdoctoral researcher at the Max Planck Institute for Intelligent Systems in Stuttgart, Germany. She completed her Ph.D. in Chemistry from Worcester Polytechnic Institute in 2014. Read more about Morgan’s research publications here or you can follow her on Twitter @morg368.
Follow the latest Soft Matter publications and updates on Twitter @softmatter or on Facebook