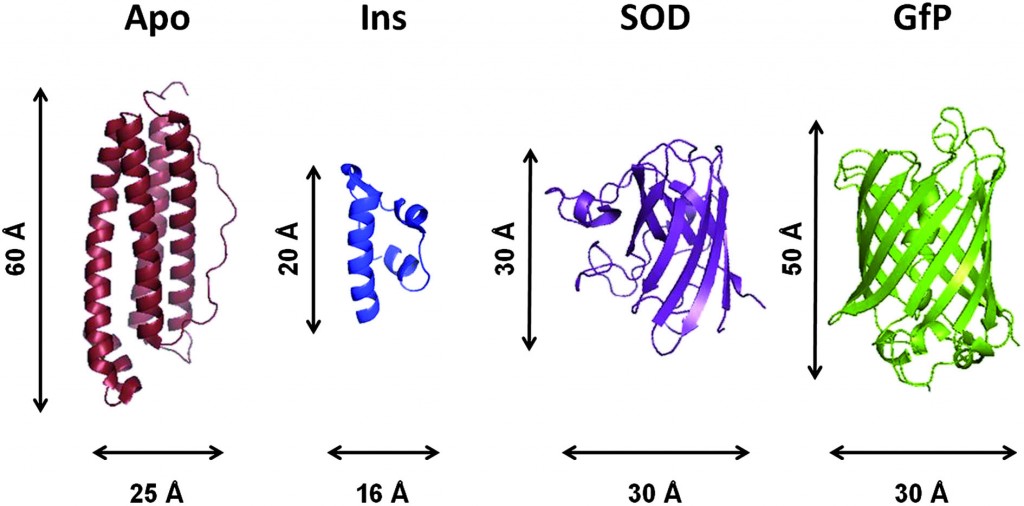
The dynamics in protein folding on the slower and larger length scale are facilitated by atomic fluctuations in the pico- and nano-second time scale. To understand the complexity of biomacromolecular materials the details of the structure-dynamics-function relationships at these scales must be analysed.
In this hot paper communication Telling and colleagues report the systematic study of macromolecular dynamics in green fluorescent protein, superoxide dismutase and insulin. The authors use neutron spectroscopy as a non-destructive and selective technique in the study, specifically elastic fixed window scattering and the newly developed technique of inelastic fixed window scattering. They find that the proteins show very different structures, but have similar methyl group compositions.
Lyophilised protein dynamics: more than just methyls?
Soft Matter, 2012, 8, 9529. DOI: 10.1039/c2sm26540k (free to read for a short time)
Follow the latest journal news on Twitter @softmatter or go to our Facebook page.
To keep up-to-date with all the latest research, sign-up to our RSS feed or Table of contents alert.