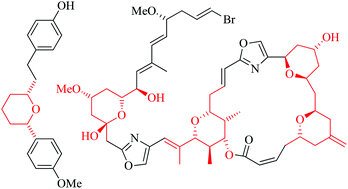
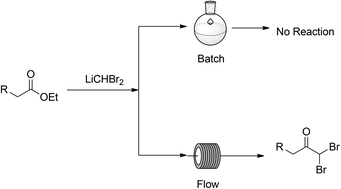
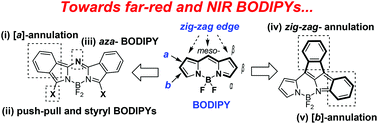
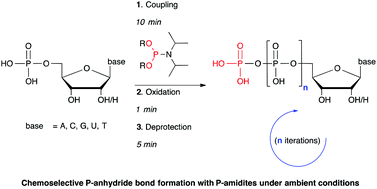
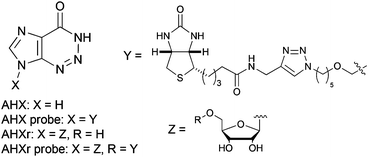
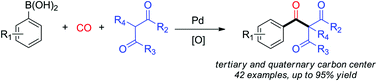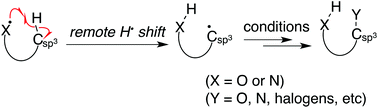Polyamine modification by acrolein exclusively produces 1,5-diazacyclooctanes: a previously unrecognized mechanism for acrolein-mediated oxidative stress
Ayumi Tsutsui, Rie Imamaki, Shinobu Kitazume, Shinya Hanashima, Yoshiki Yamaguchi, Masato Kaneda, Shinya Oishi, Nobutaka Fujii, Almira Kurbangalieva, Naoyuki Taniguchi and Katsunori Tanaka
Org. Biomol. Chem., 2014, Advance Article
DOI: 10.1039/C4OB00761A
Free to access until 18th July 2014
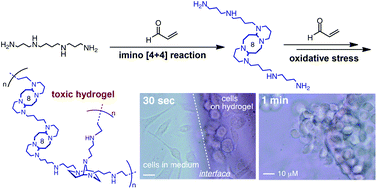
Catalytic functionalization of tertiary alcohols to fully substituted carbon centres
Long Chen, Xiao-Ping Yin, Cui-Hong Wang and Jian Zhou
Org. Biomol. Chem., 2014, Advance Article
DOI: 10.1039/C4OB00718B
Free to access until 18th July 2014
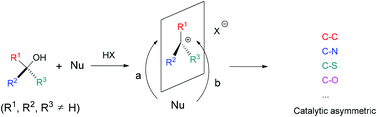
Pd-catalyzed carbonylation for the construction of tertiary and quaternary carbon centers with sp3 carbon partners
Wei Lu, Yang Li, Chao Wang, Dong Xue, Jian-Gang Chen and Jianliang Xiao
Org. Biomol. Chem., 2014, Advance Article
DOI: 10.1039/C4OB00568F
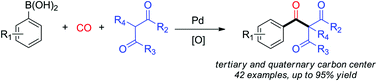
Free to access until 18th July 2014
Asymmetric synthesis of substituted NH-piperidines from chiral amines
Lekh Nath Gautam, Yijin Su, Novruz G. Akhmedov, Jeffrey L. Petersen and Xiaodong Shi
Org. Biomol. Chem., 2014, Advance Article
DOI: 10.1039/C4OB00657G
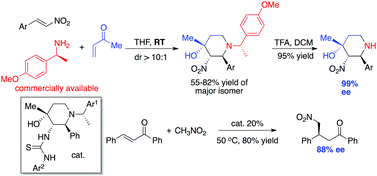
Free to access until 18th July 2014
Evaluation of 4-substituted styrenes as functional monomers for the synthesis of theophylline-specific molecularly imprinted polymers
Hazit Zayas, Clovia I. Holdsworth, Michael C. Bowyer and Adam McCluskey
Org. Biomol. Chem., 2014, Advance Article
DOI: 10.1039/C4OB00517A
Free to access until 18th July 2014
Synthesis of phosphaisocoumarin amidates via DIBAL-H-mediated selective amidation of phosphaisocoumarin esters
Yu-Juan Guo, Pei-Jiang Chen, Bo Wang and Ai-Yun Peng
Org. Biomol. Chem., 2014, Advance Article
DOI: 10.1039/C4OB00663A
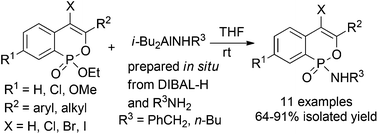
Free to access until 18th July 2014
In(OTf)3-catalysed one-pot versatile pyrrole synthesis through domino annulation of α-oxoketene-N,S-acetals with nitroolefins
Abhijeet Srivastava, Gaurav Shukla, Anugula Nagaraju, Girijesh Kumar Verma, Keshav Raghuvanshi, Raymond C. F. Jones and Maya Shankar Singh
Org. Biomol. Chem., 2014, Advance Article
DOI: 10.1039/C4OB00781F
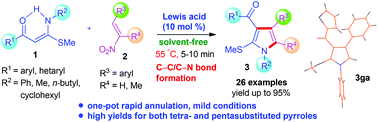
Free to access until 18th July 2014
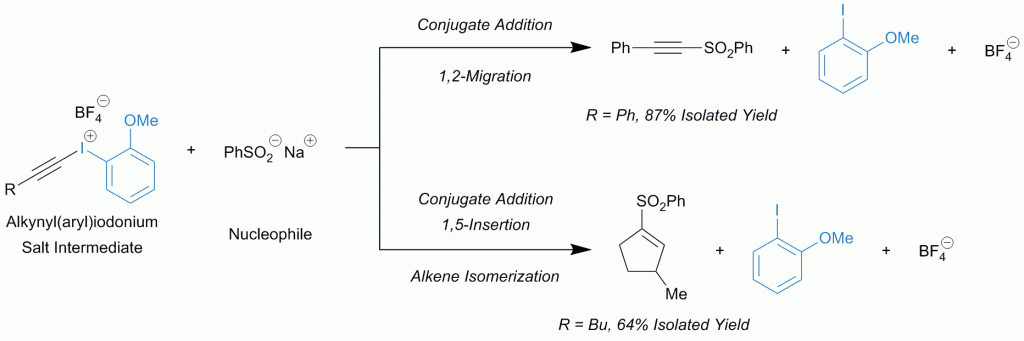
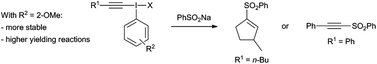
Improving alkynyl(aryl)iodonium salts: 2-anisyl as a superior aryl group
David J. Hamnett and Wesley J. Moran
Org. Biomol. Chem., 2014,12, 4156-4162
DOI: 10.1039/C4OB00556B
Free to access until 4th July 2014
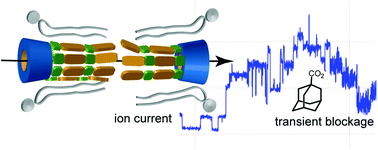
Recent development of two-photon fluorescent probes for bioimaging
Dokyoung Kim, Hye Gun Ryu and Kyo Han Ahn
Org. Biomol. Chem., 2014, Advance Article
DOI: 10.1039/C4OB00431K

Free to access until 4th July 2014
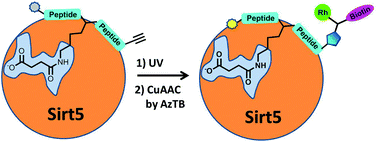
A succinyl lysine-based photo-cross-linking peptide probe for Sirtuin 5
Karunakaran A. Kalesh and Edward W. Tate
Org. Biomol. Chem., 2014,12, 4310-4313
DOI: 10.1039/C4OB00773E
Free to access until 4th July 2014
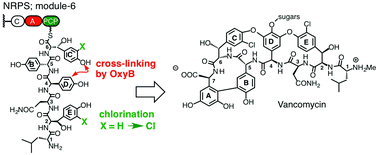
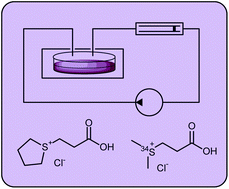
Marine bacteria from the Roseobacter clade produce sulfur volatiles via amino acid and dimethylsulfoniopropionate catabolism
Nelson L. Brock, Markus Menke, Tim A. Klapschinski and Jeroen S. Dickschat
Org. Biomol. Chem., 2014,12, 4318-4323
DOI: 10.1039/C4OB00719K
Free to access until 4th July 2014
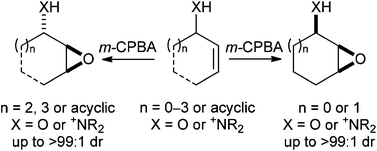
Hydrogen bond directed epoxidation: diastereoselective olefinic oxidation of allylic alcohols and amines
Stephen G. Davies, Ai M. Fletcher and James E. Thomson
Org. Biomol. Chem., 2014, Advance Article
DOI: 10.1039/C4OB00616J
Free to access until 4th July 2014
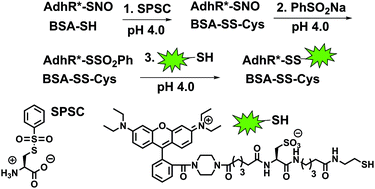
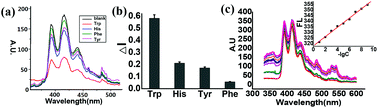
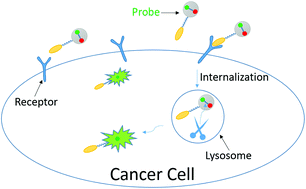
An intracellularly activatable, fluorogenic probe for cancer imaging
Ruisong Tian, Mingjie Li, Jin Wang, Min Yu, Xiuqi Kong, Yupeng Feng, Zeming Chen, Yuxi Li, Weiqiang Huang, Wenjie Wu and Zhangyong Hong
Org. Biomol. Chem., 2014, Advance Article
DOI: 10.1039/C4OB00297K
Free to access until 4th July 2014
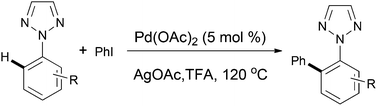
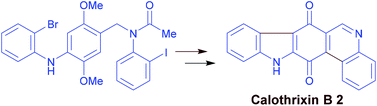
Palladium mediated intramolecular multiple C–X/C–H cross coupling and C–H activation: synthesis of carbazole alkaloids calothrixin B and murrayaquinone A
Srinivasan A. Kaliyaperumal, Shyamapada Banerjee and Syam Kumar U. K.
Org. Biomol. Chem., 2014, Advance Article
DOI: 10.1039/C4OB00493K
Free to access until 4th July 2014
Comments Off on Recent HOT Organic & Biomolecular Chemistry articles