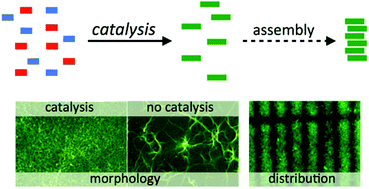
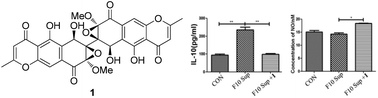
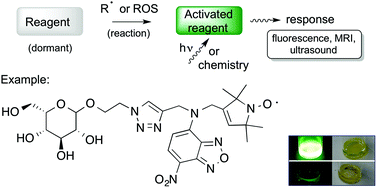
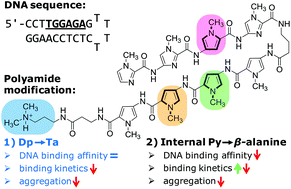
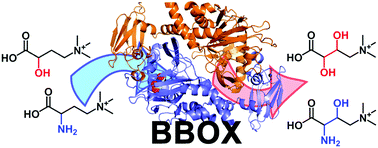The Ugi reaction was one of the very first multicomponent reactions exploited to develop chemical libraries, which further extended to combinatorial, solid phase and flow synthesis for evolving new lead structures of active agents. Implementing the Ugi reaction in combination with other organic reactions enlarges the chemical diversity of possible products.
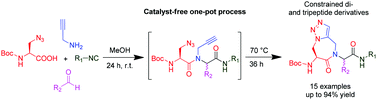
Researchers at Vrije University Brussel, led by Steven Ballet, have made a step forward by executing a one-pot Ugi-Huisgen tandem reaction and found an alternative high atom economy pathway for the synthesis of conformationally constrained, amino-triazoloazepinone-containing di- and tripeptides.
Strategically, they have used an unconventional azide containing acid, and alkyne amine along with traditional aldehydes and isocyanides. By using equimolar reactants with very mild reaction conditions, they were able to observe total conversion to the linear Ugi-compounds equipped with azide and alkyne functionality. Furthermore, they have cyclised the resulting compounds into the respective triazoles, by performing a catalyst-free Huisgen cycloaddition reaction in a sealed tube.
Variation in substitution patterns and tolerance of a large array of aldehydes in this methodology will open a new avenue for the synthesis of various building blocks to be used in peptidomimetics with a wide range of applications.
Find out more in their Communication:
Efficient synthesis of conformationally constrained, amino-triazoloazepinone-containing di- and tripeptides via a one-pot Ugi–Huisgen tandem reaction
T. M. A. Barlow, M. Jida, D. Tourwéa and S. Ballet
DOI: 10.1039/C4OB01381F













![Relative contractile motion of the rings in a switchable palindromic [3]rotaxane in aqueous solution driven by radical-pairing interactions](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C4OB01228C)