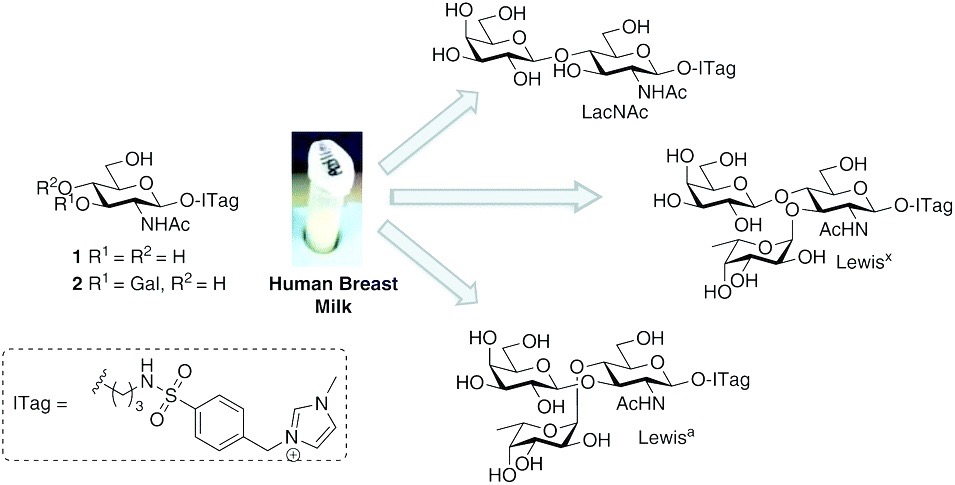
Complex coacervation refers to a liquid-liquid phase separation that arises from the association of oppositely charged polyelectrolytes in water. It is a widely used laboratory technique and coacervate-based materials have extensive application in the food and cosmetic industries as well as drug delivery and the development of biomedical adhesives.
 Under favourable conditions, the coalescence of coacervate droplets leads to a separation of a system into two liquid layers: a polymer-rich coacervate phase in equilibrium with a polymer poor supernatant. Coacervation is entropically driven and occurs through an initial electrostatic attraction between oppositely charged molecules followed by the release of counterions and rearrangement of water molecules. However, our understanding of factors that control self-assembly and stability at the molecular level remains limited.
Under favourable conditions, the coalescence of coacervate droplets leads to a separation of a system into two liquid layers: a polymer-rich coacervate phase in equilibrium with a polymer poor supernatant. Coacervation is entropically driven and occurs through an initial electrostatic attraction between oppositely charged molecules followed by the release of counterions and rearrangement of water molecules. However, our understanding of factors that control self-assembly and stability at the molecular level remains limited.
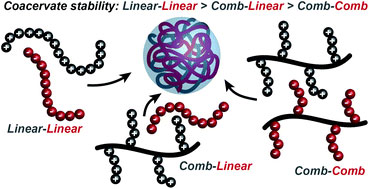
In the past, many studies have focused on how the chemical nature of a polymer affects coacervation without considering the effect of polymer architecture. In a collaborative study recently published in OBC, Prof. Sarah Perry and Prof. Todd Emerick et al., of the University of Massachusetts Amherst investigate the effect of branching in polypeptide-based comb polymers on the self-assembly and stability of complex coacervates.
In comparison to branched copolymers, the interaction of oppositely charged linear copolymers to form charge-neutral coacervate complexes is understandably straightforward. However, the extent to which a mismatched polymer architecture would alter coalescence is relatively unclear and a question that Perry and Emerick sought to answer.
The self-assembly and stability of complex coacervates resulting from oppositely charged linear polymers, linear and comb polymer and two comb polymers (see Figure) were determined/compared through turbidity measurements, optical microscopy and Monte Carlo simulations. Ultimately, it was observed that the comb structure did not form coacervates as the branched structure prevents cooperative interactions between oppositely charged polymer pairs and releases fewer counterions, leading to a weaker driving force for coacervation.
This study provides insight to the role that polymer architecture plays on complex coacervation and highlights the need to develop a detailed and predictable understanding of molecular level effects of polymer chemistry and architecture in coacervate formation.
To find out more see:
The effect of comb architecture on complex coacervation
Brandon M. Johnston, 10.1039/C7OB01314K
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at the University of Toronto. Her research is centred on the synthesis of kinetically amphoteric building blocks which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.
















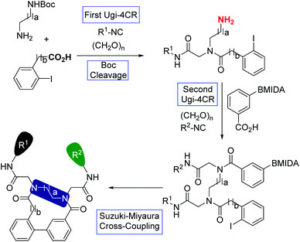 Developing efficient protocols for the synthesis of macrocycle-containing molecules has been a longstanding challenge in chemistry. In a recent OBC publication, collaborative efforts between Prof.
Developing efficient protocols for the synthesis of macrocycle-containing molecules has been a longstanding challenge in chemistry. In a recent OBC publication, collaborative efforts between Prof.