Synthesis and binding studies of novel di-substituted phenanthroline compounds with genomic promoter and human telomeric DNA G-quadruplexes
Chunying Wei, Yanbo Wang, Meiying Zhang
DOI: 10.1039/C3OB27426H
Researchers in China have developed a series of phenanthroline molecules that interact with human telomeric DNA G-quadruplexes, which are promising targets in anti-cancer drug discovery.
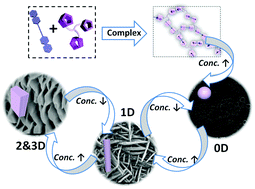
 G-quadruplexes are guanine-rich nucleic acid sequences. The guanine bases can hydrogen bond to form tetrads, and these tetrads can then stack up to form a G-quadruplex. They are significant in telomeres as their formation inhibits the telomerase enzyme, which is implicated in the onset of around 85% of cancers.
G-quadruplexes are guanine-rich nucleic acid sequences. The guanine bases can hydrogen bond to form tetrads, and these tetrads can then stack up to form a G-quadruplex. They are significant in telomeres as their formation inhibits the telomerase enzyme, which is implicated in the onset of around 85% of cancers.
G-quadruplexes are also increasingly being found in non-telomeric parts of the chromatid, such as genomic promoter regions. Their presence and purpose is still not fully understood although they have also been implicated in cell aging and the formation of cancerous cells. For these reasons, G-quadruplexes are pretty promising targets for drug discovery.
Phenanthrolines can stabilise telomeric G-quadruplexes, and some have shown excellent inhibition of the telomerase enzyme. Before now, interactions between phenanthrolines and promoter G-quadruplexes have been relatively unstudied.
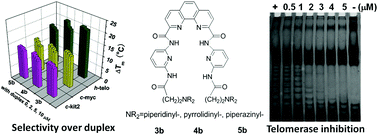
In this HOT article, Chunying Wei and co-workers at the Key Laboratory of Chemical Biology and Molecular Engineering at Shanxi University have synthesised six novel di-substituted phenanthroline molecules. They have also probed their interaction with human telomere and promoter G-quadruplexes. They have found that the compounds significantly inhibit the telomerase enzyme at low micromolar concentrations.
It is hoped that these molecules will aid and inspire the design of new anti-cancer drugs.
Annabella Newton is an organic chemist based in Melbourne, Australia. Until recently, she was a postdoctoral researcher and she has just started work as a trainee patent attorney with Phillips Ormonde Fitzpatrick.














![Direct arylation of substituted imidazo[4,5-b]pyridines Direct C2-H arylation of 6- and 7-substituted imidazo[4,5-b]pyridines](https://blogs.rsc.org/ob/files/2013/03/GA4.gif)

![Synthesis of helically twisted [1 + 1]macrocycles assisted by amidinium–carboxylate salt bridges and control of their chiroptical properties](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C2OB27054D)