In this HOT paper, K. C. Nicolaou and co-workers have developed a series of taxol analogues which show potent activity against several cancerous cell lines.
The fight against cancer is one of the hottest areas of drug discovery. Despite this, there is still a shortfall in treatment options and the disease is on the rise. The development of safer and more selective drugs is therefore required.
Paclitaxel (taxol) and docetaxel are two of the most highly successful anti-cancer drugs and much research has been performed focused on their synthesis and structure. K C Nicolaou and his group at Scripps are well-versed in the construction of paclitaxel; the group published one of the first total syntheses of this complex molecule in 1994.
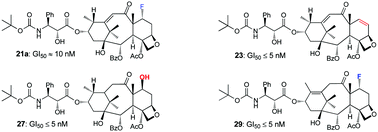
10-Deacetylbaccatin III is an advanced synthetic precursor to paclitaxel and shares many its core structural features. Thanks to the synthetic efforts of recent years, it’s also readily accessible and therefore it provides an interesting starting point for further structure-activity relationship studies. Nicolaou and Valiulin have found that, upon treatment with diethylaminosulfur trifluoride (DAST), 10-deacetylbaccatin III undergoes a nifty vinylogous pinacolpinacolone rearrangement leading to a new enone structure and its fluorinated analogue.
Nicolaou and Valiulin have capitalised on this discovery and have prepared an small library of structurally analogous taxoids using this reaction. The library of analogues was submitted to screening program run by the National Cancer Institute (NCI) where the compounds were evaluated against 60 different cancerous cells lines. Several of the taxoids showed significant potency against numerous tumour cell lines. This study has revealed important information regarding the structure-activity relationship of the taxoid family of molecules. It has also produced some promising potential leads for new anti-cancer drugs.
Synthesis and Biological Evaluation of New Paclitaxel Analogs and Discovery of Potent Antitumor Agents
Kyriacos C. Nicolaou and Roman A. Valiulin
DOI: 10.1039/C3OB40654G
Free to access for 4 weeks











![cholera-toxin-inhibitor_c3ob40515j_300[1]](https://blogs.rsc.org/ob/files/2013/05/cholera-toxin-inhibitor_c3ob40515j_3001.jpg)