We are delighted to have received the authors’ comments from Christopher S. P. McErlean on the recently published HOT article “Revitalizing the aromatic aza-Claisen rearrangement: implications for the mechanism of ‘on-water’ catalysis”.
“Chemistry creates. It is the only discipline in which you can design or imagine a new entity and then go away and build it. Of course, it is always easier said than done, so synthetic organic chemists spend a good deal of their time exploring ways to build molecules more efficiently. That’s what the McErlean Research Group does at the University of Sydney. We are primarily focused on making synthesis easier.
Every undergraduate chemist knows that the aromatic Claisen rearrangement is a great way to build functionalized phenols. The reaction is so prevalent in heterocycle synthesis that we tend not to reference the original work – it is assumed knowledge. Our group envisaged that the aromatic aza-Claisen rearrangement would be an equally powerful way to make functionalised anilines, but the known reaction conditions are just too forcing to be useful.
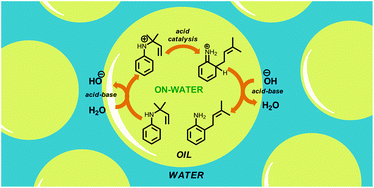
“On-water” reactions rely on interfacial water to catalyze chemical transformations. Exactly how the interfacial water does this has been the source of much recent debate. Most of the protagonists have relied on computationally derived evidence to support one theory or another. We thought that we could kill two birds with one stone by using on-water catalysis to facilitate the aromatic aza-Claisen rearrangement and probe the kinetics of this transformation.
The outcomes of our work are twofold: an easy way to build functionalised naphthylamines and anilines, and experimental evidence that supports the acid-catalyzed nature of on water chemistry.
We hope that chemists employ this operationally simple synthetic tool to build life-changing molecules, and we hope that our results will spur more experimental chemists to provide hard data regarding the on-water phenomenon.”
This Communication is free to access for the next 4 weeks!
Revitalizing the aromatic aza-Claisen rearrangement: implications for the mechanism of ‘on-water’ catalysis
Kaitlin D. Beare and Christopher S. P. McErlean
DOI: 10.1039/C3OB40118A
Interested in Christopher S. P. McErlean‘s research? Why not also read these recently published articles:
An in-water, on-water domino process for synthesis
Norcott, Philip; Spielman, Calan; McErlean, Christopher S. P.
Green Chem., 2012, 14, 605.
DOI: 10.1039/c2gc16259h
Total synthesis of C-19 lipid diols containing a 2,5-disubstituted-3-oxygenated tetrahydrofuran
Nesbitt, Caroline L.; McErlean, Christopher S. P.
Org. Biomol. Chem., 2011, 9, 2198.
DOI: 10.1039/c0ob00754d